With two novel immunotherapies approved and many more in the pipeline, is it time to announce that a new treatment modality has emerged?
A class of therapy that has long held promise for treating cancer patients may finally have come of age. Immunotherapy, or biological therapy to give the broadest term, can use the body’s immune system in a wide variety of ways, by directly attacking tumours, controlling factors that allow tumour growth, using vaccines to prevent but also to treat cancers, helping repair damage from other treatments, and more. Now, after more than a century in which the tantalising possibilities for harnessing the immune system to fight cancer have commanded sporadic interest but only limited success, a flood of recent research findings have earned their place in top journals and ASCO presentations, with promising trial results and a big pipeline of new agents.
Investment analysts, never slow to spot trends, suggest we might be approaching a tipping point – the number of ASCO abstracts on this topic more than doubled over the last two years, reaching more than 300 this year. Researchers now talk of the ‘end game’ being in sight for immunotherapy, though there is still a long way to go. The excitement that is building was triggered in no small part by the approval in 2010 by the US regulatory body, the FDA, of the first vaccine therapy, sipuleucel T (Provenge) for advanced prostate cancer, followed last year by ipilimumab (Yervoy) for metastatic melanoma, which is an ‘immune-targeted’, or stimulatory, antibody agent.
Meanwhile the wider public are being primed about the possibility of a major breakthrough. This April, The New Yorker, a literary magazine, carried a story titled ‘The T-cell army’, in which writer Jerome Groopman traces the early history of immunotherapy back to William Coley, a surgeon in New York, who stumbled on a case where a streptococcal infection seemed to help a sarcoma patient eliminate his cancer, and then tried to replicate it.
T cells – a type of lymphocyte, or white blood cell, found in the blood and other parts of the body –work mainly by producing proteins which allow immune system cells to communicate with each other, and which can also attack foreign or cancerous cells. It is the discovery of one type of T cell that produces a protein called cytotoxic T-lymphocyte antigen-4, or CTLA-4, that Groopman describes in detail, as it led to the development of Yervoy.
The breakthrough moment came when Jim Allison, head of tumour immunology at Memorial Sloan Kettering, and colleagues, spotted that the mechanism worked in the opposite way to what had been believed – CTLA-4 had to be blocked, not stimulated. It then took a while before they finally persuaded a drug company to take up the approach.
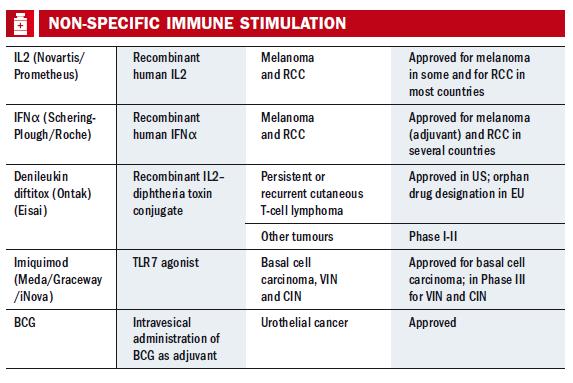
Drug companies could be forgiven for being cautious, given the long history of difficulties. Interleukin-2 (IL-2) and interferon-α – the main stays of attempts at improving immune responses introduced in the 1980s – both have significant toxicity and limited evidence for overall survival. The past few years have also seen high profile failures of companies such as CancerVax and Cell Genesys. But this unpromising picture has changed dramatically over the last two years.
There are two reasons why immunotherapy has now gained such traction, says Christian Ottensmeier, professor of experimental cancer medicine at the University of Southampton in the UK. “We’ve had a poor understanding of how the immune system works – but that is changing rapidly with work on chronic infectious diseases such as malaria, TB and HIV, as well as cancer – they are cross-fertilising and similar questions are being asked. Second, our tools are much better – we had quite rudimentary ways of looking at immune response, but in the past 10 years there has been an explosion in what we can deliver for measuring immunity. Being able to measure in more detail the different facets of the immune system, and in ways that others can reproduce, is a major contributor to the field.”
“In the past 10 years there has been an explosion in what we can deliver for measuring immunity.”
As a result, he adds, we are now in a better position to study one of the least explored approaches that have the potential to improve outcomes for cancer patients. “There is also a rapidly accumulating body of evidence to indicate that immune attack on cancer is critical, and looking at it just numerically in the tissue is a very powerful tool for predicting outcomes – so there is a strong argument that improving outcomes can be achieved through improving immune responses. And there is a rapidly growing number of randomised phase II and III studies that show this is actually the case. The field has changed in just two years from having little to offer to being a ‘grown up’ treatment.”
“The field has changed in just two years from having little to offer to being a ‘grown up’ treatment.”
Joost Lesterhuis, a medical oncologist and researcher at Radboud University Nijmegen Medical Centre, in the Netherlands, says that the basic research community had not lost faith with immunotherapy. “There have been a lot of papers in preclinical and translational research journals – there was a difference in perception about what immunotherapy can do between clinicians and laboratory scientists. ”A string of high-profile negative trials, in particular with vaccines, has not helped of course, but there has been steady refinement of animal models and a flow of small human studies that paved the way for the impact we see now, with the standout sector being the novel immune-targeted agents such as Yervoy, says Lesterhuis.
He outlines some of the key advantages of immunotherapy. “It can be very specific – T cells can be directed against tumour cells, in theory without causing any damage to surrounding tissue. The immune system also has a memory, so if you induce a response, in general it is long-lasting and can be quite potent – we’ve seen instances in people with high tumour burden, although people with less tumour tend to respond better.
“And immunotherapy is additive – the old idea was that you should go for one treatment or another – now we are moving in the near future to giving immunotherapy with or on top of other therapies.”
Strategies in immunotherapy
Immune checkpoint blockade
Among the big news stories at ASCO this year were trials of two agents in the class of immune stimulatory antibodies, targeting the PD-1 (programmed cell death) and PD-L1 (programmed cell death ligand) proteins. The aim is to block pathways that shield cancer cells from the immune system, and the agents have been trialled not only in melanoma and kidney cancer, which have long been candidates for immunotherapy, but also in non-small-cell lung cancer (NSCLC), with promising results in these hard-to-treat advanced tumours. Both agents are made by Bristol- Myers Squibb (BMS), which also makes Yervoy.
The reason Yervoy has gained so much attention is straightforward, says Lesterhuis: it is the first drug to show survival benefit in a phase III trial in advanced melanoma (although the BRAF inhibitor, vemurafenib, has competed for attention, and the two are being trialled together now). “It wasn’t that most patients were cured – they weren’t – but it was the first positive story to tell about melanoma, and there were dramatic responses in a minority, and it was the spark that made a lot of people enthusiastic again.”
This type of immunotherapy is progressing very rapidly, he adds, because clinical data are becoming readily available, and because it is not a patient tailored approach. “It’s just an antibody against a surface molecule on T cells.”
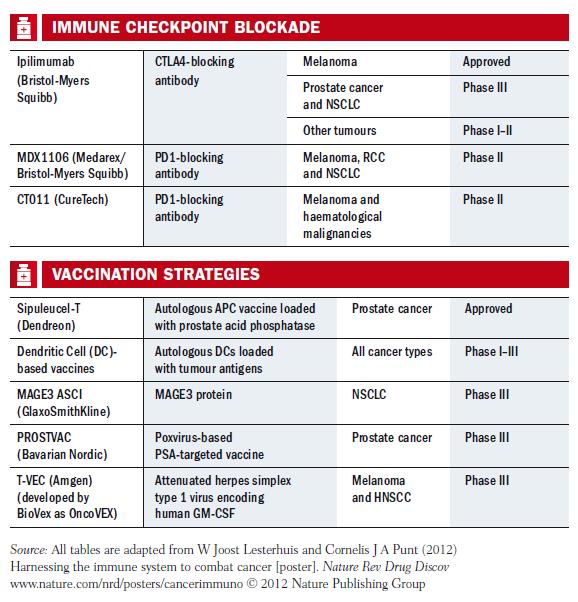 Therapeutic vaccines
Therapeutic vaccines
Ottensmeier points out that there are well-established immune treatments, such as bone marrow and stem cell transplantation in haematological malignancies, but the spectrum of treatments is opening up widely now. In addition to agents such as Yervoy, he considers that therapeutic vaccines such as Provenge are becoming valid treatment options.
His opinion is shared by Lesterhuis, who co-wrote a review paper published August 2011 in Nature Reviews Drug Discovery, where they argue that therapeutic vaccines are more widely applicable than preventive ones, as most human cancers have several causal agents. Such vaccines can be developed in a variety of ways, using viruses, proteins, DNA, peptides, dendritic cells and so on. Some of these strategies are showing promise, they say, but most have failed. One important lesson they highlight from past mistakes is that enough time must be allowed to judge the potential impact of a vaccine in early-stage trials.
Despite the many setbacks, there is now a pipeline of candidates in phase III, such as GlaxoSmithKline’s DERMA and MAGRIT trials for non small-cell lung cancer and melanoma that target the MAGE-A3 antigen, and Amgen’s talimogene laherparepvec (T-VEC), an engineered herpes virus that also targets melanoma. Ottensmeier adds that there are at least five randomised vaccine trials for lung cancer that are well worth watching.
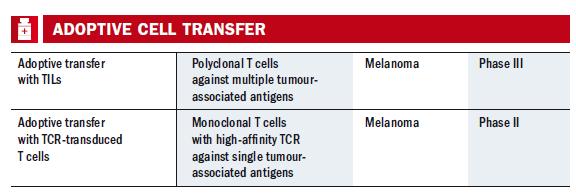 Lab-grown T cells
Lab-grown T cells
Another interesting approach mentioned by Lesterhuis is adoptive T cell therapy. While the immune-targeted therapies and vaccines aim to induce or boost the body’s existing responses to tumours, adoptive T cell therapies culture large numbers – potentially billions – of tumour-specific Tcells in the lab, and infuse these into the patient. This strategy was developed in the US (for example in a trial in 2002 with advanced melanoma, although early work goes back to the 1980s), and has now started to become available for a few melanoma patients in Europe, at centres such as the Amsterdam Cancer Institute, Copenhagen University/Herlev Hospital in Denmark, and the Christie in Manchester, UK. The T cells can also be derived from blood or can be genetically modified, but in the main melanoma trials they come from the tumour – a treatment known as TIL (tumour infiltrating lymphocyte).
 The role of chemotherapy
The role of chemotherapy
The case is now being made that immunotherapy deserves to be classed as a distinct treatment modality, to rank alongside chemotherapy, hormonal therapy and the new targeted therapies. But as usual with cancer, things are not so clear-cut. Take chemotherapy – as Ottensmeier says, “We are learning that both old and new chemotherapies that are not immunological in nature do produce immunological effects. Some think that chemotherapy is going to immuno-suppress the patient, but that’s not true for all drugs. For example, there is a recent paper in the JCO that found the number of immune cells in breast cancer predicted more benefit from chemotherapy. It means that when we have been focusing only on the cytotoxics in terms of poisoning cancer cells, it may be much more complex than that.” The benefit from both chemotherapy and radiotherapy, he adds, may be related to immunological factors and not primarily to the toxic effects.
“Benefit from both chemotherapy and radiotherapy, he adds, may be related to immunological factors”
This is pointing to new directions for investigation with conventional treatments and with targeted therapies to find out whether they can work in conjunction with immunological treatment. “Some are particularly good and some really bad – we need a case-bycase analysis to understand the principles,” he says.
Investigating the effects of platinum-based chemotherapies in combination with immunotherapy is Lesterhuis’s own field and he’s recently returned from a spell as a visiting scientist at the tumour immunology group at the University of Western Australia. “I’ve found that there are beneficial effects in induction of immunity, such as by activating dendritic cells, making tumour cells more susceptible to immune attack,” he says. “It feels counterintuitive because one of the side-effects of chemotherapy is immune suppression with decreased immunity to bacteria, but in recent years we have evidence that immune response to tumour antigens is not decreased and may alarm the immune system towards cancer.”
He points to another study, a phase II trial that has attracted interest, where Yervoy was combined with chemotherapy in non-small-cell lung cancer. “It showed longer progression free survival depending on the scheduling of the two drugs, and phase III is now starting. The other exciting thing is that it is in lung cancer, which was thought to be a non-immunogenic disease.” The anti-PD-1 agent has also shown some good responses in lung cancer, he adds.
Towards the end game?
But the successes so far should not disguise the many obstacles for making more progress in immunotherapy. While investigation and tools are developing fast, major gaps in knowledge remain regarding, for example, optimal dosage, scheduling and how to measure response. The rulebook for cytotoxic drugs is no good here; as Lesterhuis and colleagues point out in their review paper, maximal tolerated dose and tumour response rate have proved not to be valid as markers for immunotherapies, and there is much less – if any – correlation between drug exposure and efficacy and/or toxicity.
In particular, patients may take much longer to respond to treatments, and tumours may grow for a while. Benefits may not emerge for months or possibly years, which can leave oncologists and patients facing difficult decisions about whether to continue with treatments. Lesterhuis also notes that there can be unexpected side-effects, such as the high rate of acute renal failure that occurred in a trial of a combination therapy for kidney cancer. Generally, side-effects can be severe in immunotherapy, as found in trials of Yervoy, and can require fast medical action.
Inevitably, there are cost and regulatory issues concerning the new agents. When Provenge hit the headlines, it was on account of its price tag as much as anything else. Yervoy is not far behind, at about €85,000 for one course of infusions in the Netherlands, for example. A recently established cancer drugs fund in the UK is covering costs there, but at a ‘tear-inducing’ price, says Ottensmeier.
As with other therapies, there is a need to identify patients who will benefit. His own group presented a poster at ASCO on early work on a biomarker for gauging who might benefit from Yervoy for melanoma. Using a proprietary panel of tumour-associated antigens, they found that among patients treated with the therapy, those with pre-existing antibodies against two or more antigens were significantly more likely to survive. They concluded that the melanomas in these patients “are immunologically more visible”, and so more likely to respond to activation of immunity.
Ottensmeier says that the current focus is mainly on treating established disease, but attention will also turn more to prevention of recurrence, and primary prevention and prevention of disease development – the HPV vaccine for cervical cancer being an obvious example of a primary prevention. “And the excitement about anti- CTLA-4 is not only that it works in a small number of patients, but also that there is a group of patients who do not have recurrence after you’ve finished treatment, and it’s a paradigm shift that I think we will see with vaccines and T cell transfer as well.
“It is a result of the memory of the immune system, both in B cells, which make antibodies, and T cells. They hang around for decades – probably for life. If they are enabled to see the tumour, then they can do what our current drugs have not been able to do.”
ENLISTING 196 BILLION T CELLS TO HELP FIGHT STAGE 4 MELANOMA
When Hein Jambroers, a 47-year-old event coordinator from Roemond, a city in the southeast of the Netherlands, found a small mole on his leg that was growing, he went to his doctor, who sent him to the local hospital, where it was found to be melanoma. He then went to the university hospital in Maastricht for operations on his spleen and lymph nodes – but one year later, the cancer returned with tumours on his leg. This was summer, 2010. “I went back to Maastricht and they said they couldn’t help me as the cancer was now in my blood. I went to Rotterdam too and was told again there was no treatment. So I started Googling.”
Jambroers found the story of a Belgian woman who had been treated with ipilimumab in Brussels, and tracked her down on LinkedIn. After talking to her, and her oncologist, he was advised to see John Haanen at the National Cancer Institute (NKI) in Amsterdam, who was working on the latest melanoma trials.
“I was first put on vemurafenib [Zelboraf], the new BRAF inhibitor, and that worked well at first – a spot on my liver vanished and others shrank to a pea. But then it stopped working and tumours in my leg grew again, to egg size, within one month.” He was then offered Yervoy, which had worked in other patients in Amsterdam. “I had four infusions but it didn’t do anything – it is thought it could work after several months but there was no change in my blood work at all. I was told they were not expecting an anti-PD-1 trial before 2012.”
He had already asked about adoptive T cell lymphocytes (TIL) in 2010, but had been told there were no plans to try it. Thankfully he then got a call inviting himto be one of the first three patients in the Netherlands to undergo the treatment, under Haanen.
Jambroers says that TIL is a major procedure involving several stages and people need to be physically fit. “First I had an operation to remove some tumour to get the antigen-specific T cells, but I got an infection and had to wait another month while that was treated. I then went back to have white cells removed from my blood and had a week of chemotherapy.” That process – called lymphodepletion – is needed to eliminate competing lymphocytes and regulatory T cells. Meanwhile, the specific T cells were cultured for infusion – there were about 196 billion in his case, which he received in October last year. “I was told that 196 billion is an extremely high number of cultured cells – the results of all the worldwide TIL trials show anything over 150 billion gives a good chance of complete remission.”
But the treatment was not over – he also had four infusions of IL-2, and had to go into intensive care as his liver and kidney began to fail. A week later, he was well enough to go home. A check revealed the tumours had shrunk by 25–30%, and he was soon back at work. “After another two weeks there was a 50% shrinkage, and by Christmas therewere no active tumours – just one lump with fluid. By April this year it looked like I was cured – there was only scar tissue and nothing in my blood.” Jambroers, pictured here with his wife Varadi and daughter Jenna, has become an advocate for melanoma patients, telling his story on websites and in Dutch newspapers. There is much more about the latest treatments now available, but he feels that, even now, many doctors are still unaware of where to refer people. “I know how hard it is if you can’t find information and I’m happy to tell my story whenever I can.”