In this overview a sarcoma specialist present current evidence on the best way to manage osteosarcoma patients and looks at what has to be done to improve prognosis in this disease, where survival rates have not changed since the early 1980’s.
Osteosarcoma is a malignant mesenchymal tumour producing osteoid. It is a rare type of cancer, with an incidence of only 2–3 per million per year, occurring mostly in adolescence and affecting more males than females (in a ratio of 1.4:1). In adolescence it usually occurs in the metaphyses of long bones, usually around the knee. The problem we face is with metastases, which occur in about 90% of patients. Both primary and later metastases usually occur in the lungs and sometimes in bones, but rarely elsewhere.
In terms of imaging methods, the osseous compartment it still best visualised by conventional X-ray. This is the method of choice for bony alterations. MRI is also needed to look at the primary tumour, showing the amount of marrow involvement, the soft tissues, and the relationship to vessels and nerves, providing essentially all the information the surgeon needs. Imaging for systemic spread depends on location: X-ray for the chest and bone scans for bones. But the most valuable imaging method is a CT scan of the chest.
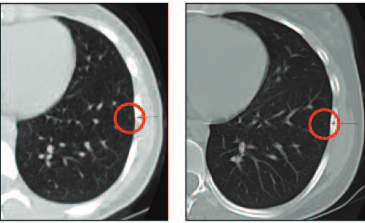
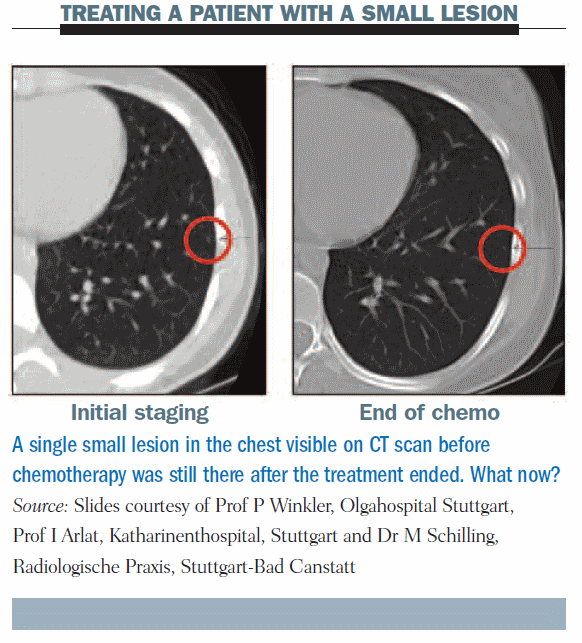
This CT scan shows initial staging in a patient who has a very tiny lesion; it is the only lesion and the rest of the CT scan is normal. At the end of chemotherapy, there was no change. How should we treat this patient? We will come back to this later.
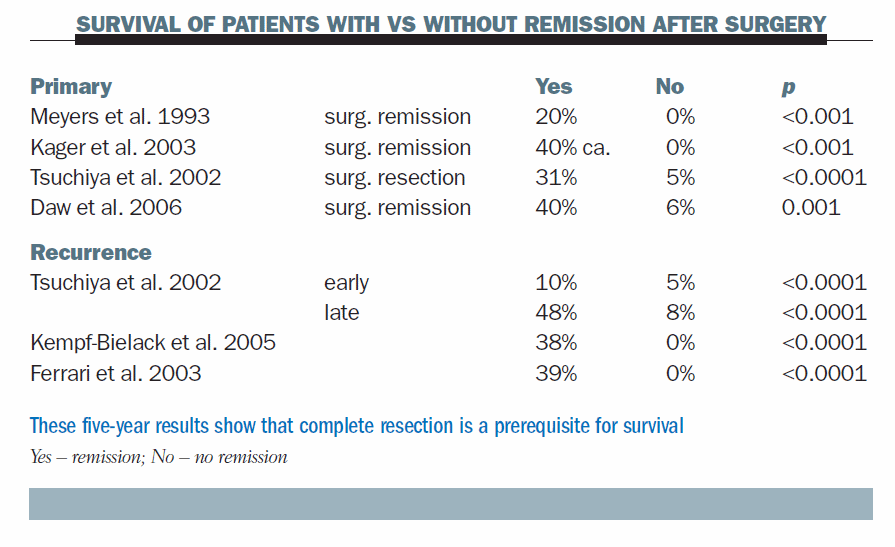
 The table below shows results of surgery in several series of patients with primary metastatic osteosarcoma, comparing those who achieved surgical remission with those who did not. In most series, patients who did not achieve a surgical remission, or whose metastases were not removed, did not usually survive for five years. The situation is similar at recurrence. Patients who do not achieve a surgical remission rarely become long-term survivors; patients who do achieve a surgical remission have a reasonable chance of long-term survival. Complete surgery is necessary for long-term survival.
The table below shows results of surgery in several series of patients with primary metastatic osteosarcoma, comparing those who achieved surgical remission with those who did not. In most series, patients who did not achieve a surgical remission, or whose metastases were not removed, did not usually survive for five years. The situation is similar at recurrence. Patients who do not achieve a surgical remission rarely become long-term survivors; patients who do achieve a surgical remission have a reasonable chance of long-term survival. Complete surgery is necessary for long-term survival.
 Identifying metastases
Identifying metastases
How well can CT tell us whether metastases are present? A very interesting joint Italian–Scandinavian study looked at 51 osteosarcoma patients with suspected metastases on CT scan. At surgery it was found that 39 had metastases while 22 did not (Ann Oncol 2001; 12:1601–04).
How can we tell whether small nodules might be metastases? Radiologists always tell us that small nodules that do not change after chemotherapy cannot be metastases, or that if they disappear they are not metastases. What the Italian–Scandinavian study found was that changes in nodule number and size during chemotherapy did not indicate whether patients really had metastases. The only factor that was significant was that a lesion smaller than 5 mm was less likely to be a metastasis than larger lesions (P=0.035). But ten of 25 patients with nodules <5 mm had metastases. So if a patient has a small nodule on CT scan, even if it is only one, and even if it does not change during therapy, it can be a metastasis.
Another study from New York looked at 28 patients who underwent 54 thoracotomies. Preoperative CT scans in all the patients showed 183 suspected nodules. At surgery, the surgeons found 329 nodules, 209 of which were osteosarcoma. This means that a CT scan had underestimated the number of lesions in 19 of the 54 patients who were referred for scanning – about one third (J Pediatr Surg 2006; 41:200–206).
My take home message for lung metastases, both primary and secondary, is that there is no perfect imaging methodology. CT is the best, but it is not perfect. You will often find more lesions than expected and should look bilaterally, even if a CT scan has shown metastases only on one side. It is essential to remove all metastases or the patient will not survive.
Surgery is usually open thoracotomy. Video-assisted thoracoscopic surgery (VATS) is not recommended because surgeons should palpate the lungs. We do a CT scan after thoracotomy and we send patients back to the thoracic surgeon if there are still metastases. I would even send them back a third time if there were still no remission.
Going back to our discussion of the patient with the very tiny lesion that did not change during chemotherapy: when she underwent thoracotomy the lesion was found to be a metastasis and she had three more that were not evident on CT scan.
Question: Could you give some indication of the potential role of adjuvant chemotherapy in patients with recurrent metastatic disease in the lungs? You have clearly outlined the role for surgery. What is the role of chemotherapy?
Answer: There are two situations. One is when lung metastases are inoperable and you cannot remove them by surgery. The largest series in inoperable metastases all have the same message: if you give chemotherapy, you can prolong survival by several months but you will not cure the patient. As a group, patients who receive chemotherapy live a few months longer. The second situation is where you do achieve surgical remission. Here the role of second-line adjuvant chemotherapy is not as clear. The two largest series are from Italy, and from our German/Austrian/Swiss group. The Italians did not find any benefit from second-line adjuvant chemotherapy. We found that freedom from second recurrence was increased by about 5–6%, which was statistically significant but not a tremendous improvement. In a patient with pulmonary metastases at recurrence, I would discuss and offer adjuvant chemotherapy, but I would never try to convince someone against their will.
Question: Does PET have a role in defining metastatic disease?
Answer: No, there is currently no role for PET in defining metastatic disease in osteosarcoma. PET will not usually pick up lung metastases that are too small to be seen by CT. Even larger lung metastases – up to about 1 cm in diameter – are sometimes not picked up by PET. Bone metastases are rather infrequent as primary metastases. You usually see them by bone scan and there are no data that indicate PET would be more sensitive. Where PET may have role is in following the osteosarcoma during preoperative chemotherapy, predicting response to preoperative chemo.
Question: What is the role of radiotherapy in lung metastases?
Answer: We have a very old study with whole-lung adjuvant chemotherapy – a randomised trial from the EORTC. This study, performed in the late 1970s, showed that if you do not give chemotherapy, then adjuvant radiotherapy to the lungs will reduce the risk of recurrence by a small amount. Adding adjuvant radiotherapy to effective chemotherapy will not add anything significant. The only role that I see for radiotherapy of lung metastases is for a limited lesion that you cannot resect. I would discuss with the radiotherapist whether it could be irradiated.
Surgery after primary tumour
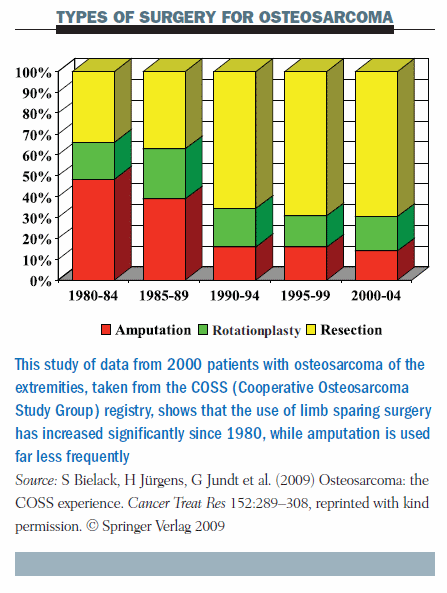
The bar chart below shows the types of surgery used in a large multicentre study by five-year intervals for 2000 extremity osteosarcomas. In the 1980s, limb salvage (shown in yellow) was used in about one-third of patients, and the other two-thirds had either amputation or rotationplasty. The proportion of amputations has since dropped dramatically. Most patients with extremity osteosarcomas treated since 2000 went on to have limb salvage (Cancer Treat Res 2009; 152:289–308).
 Local recurrence
Local recurrence
Our group looked at recurrence in 1702 osteosarcoma patients; 576 developed recurrences and 75 of these had local recurrence. Forty-four had local recurrence only and their five-year survival was 26%; 31 had local recurrence combined with metastases and their five-year survival rate was only 7% (JCO 2005; 23:559–568). The results show the importance of avoiding local recurrence.
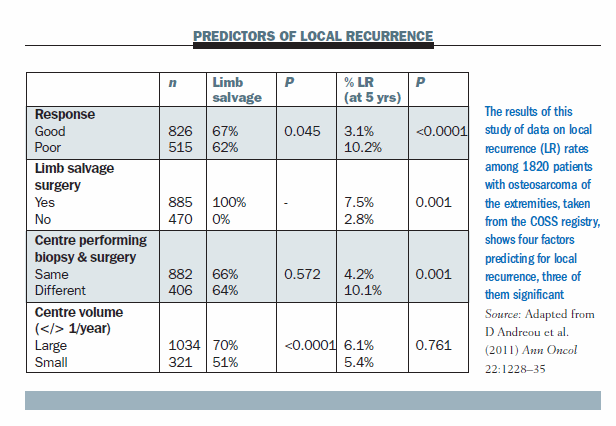
A study conducted by our group using data from the COSS (Cooperative Osteosarcoma Study Group) registry (Ann Oncol 2011; 22:1228–35) shows there are three predictive factors for local recurrence.
Tumour response to chemo-therapy. The local recurrence rate for patients who had poor response to chemotherapy was 10% while the local recurrence rate for patients who had a good response (less than 10% viability of the tumour) was only 3%. So, chemotherapy clearly has a role in local control.
Limb salvage surgery. There was a slightly higher local recurrence rate for patients who had limb salvage. In our experience, the local recurrence rate was 2.8% in 470 patients who had amputations and 7.5% in 885 patients having limb salvage. So, there is a higher local recurrence rate for those patients having limb salvage.
Location for the biopsy and the surgery. Patients who had to move from the centre that did the biopsy to another for surgery had significantly higher local failure rates. This may be because often the biopsy was not performed in a manner conducive to definitive surgery and may have contaminated the wound more than desired.
Surgical volumes. I would have expected that patients who had surgery at a centre doing more surgery would have a lower local recurrence rate than small centres not doing as many operations, but in our series they had identical local recurrence rates, so surgical volume is not a predictive factor. However, our figures did show that the large centres did limb salvage in 70% of patients, while the small centres did this in only 50% of patients. So, while the local recurrence rates are the same at large and small centres, there is a higher rate of amputation if the surgeon is not very familiar with sarcoma surgery.
 Local therapy for inoperable sites
Local therapy for inoperable sites
Inoperable sites include many axial osteosarcomas and metastases that can’t be reached by surgery. It is usually said that radiotherapy does not work for osteosarcoma. Is that really true? There are a couple of interesting publications on this. DeLaney and colleagues from the US looked at 41 patients with osteosarcoma and inoperable lesions: 27 primaries, 10 local recurrences and four metastases. They were given radiation and some chemotherapy. The local control rate was 68% at five years, with relatively high doses of radiation (median 66 Gy). It was 78% for patients who had gross or subtotal resection together with radiotherapy and 40% for those who had biopsy only (Int J Radiat Oncol Biol Phys 2005; 61:492–498).
We did a retrospective analysis of the COSS registry data for 100 patients with 66 primary tumours, 11 local recurrences and 23 metastases. Radiation doses were also relatively high (median 56 Gy) and all patients had chemotherapy. The local control rate was 30% at five years in this highly heterogeneous multicentre cohort: 48% for surgery plus radiotherapy, 22% for radiotherapy alone, 40% for primary tumours, 17% for local recurrences and 0% for metastases (Cancer Treat Res 2009; 152:147–164).
The information we can take out of this is that radiotherapy can work for selected osteosarcoma lesions that are not operable. The finding that it works better if you take out large parts of the tumour – debulking – is interesting, as is the finding that it works better for primary tumours than for recurrences or metastases. I think this is because radiotherapy works best if you give it together with effective chemotherapy, and we have effective chemotherapy for primary osteosarcoma but not for recurrences.
The take home message for local therapy is: operate, operate, operate. Surgery is the most important local therapy. You need a good surgeon who knows how to achieve adequate margins. Limb salvage is often feasible. The risk of local recurrence can be reduced by getting four things right:
- good imaging, because the surgeon needs to know where to cut
- smart planning between all disciplines
- good chemotherapy to devitalise the tumour as much as possible, and
- good surgery.
Radiotherapy may be an option for selected inoperable lesions. There are some studies with proton or heavy ion radiotherapy that may help to define whether these innovative radiation techniques can be more effective than conventional radiotherapy.
Question: What do you do if you have a residual disease following surgery? What is the role for amputation in the modern era?
Answer: If you do not achieve adequate margins with limb salvage, then you should amputate. I think you also need to think about limb amputation and particularly rotationplasty for tumours in very small children, because growing endoprostheses are tedious for young children in their lives.
Chemotherapy
Chemotherapy for osteosarcoma was started and evaluated in the 1970s with three drugs: high-dose methotrexate, doxorubicin, and cisplatin. Ifosfamide was added in the 1980s. These drugs are still being used today. Adjuvant combination regimens were introduced in the late 1970s and early 1980s and preoperative (neo-adjuvant) chemotherapy has been used since the early 1980s.
Timing of chemotherapy in relation to surgery
We all think that you have to give preoperative chemotherapy, but is this true? Results of a randomised study by the Pediatric Oncology Group in 100 patients (JCO 2003; 21:1574–80) did not show a significant difference in five-year event-free survival between patients who had immediate surgery and those who had their surgery after a course of preoperative chemotherapy (69% vs 61%). Similarly our experience for the COSS group, looked at retrospectively but with larger patient numbers, shows that patients who had delayed surgery after preoperative chemotherapy had the same prognosis (54.4%) as those who had surgery first and chemotherapy after (59.9%) (JCO 2002; 20:776–790). We can conclude that, if you give the same total amount of chemotherapy, it probably does not matter much when you perform surgery in terms of survival.
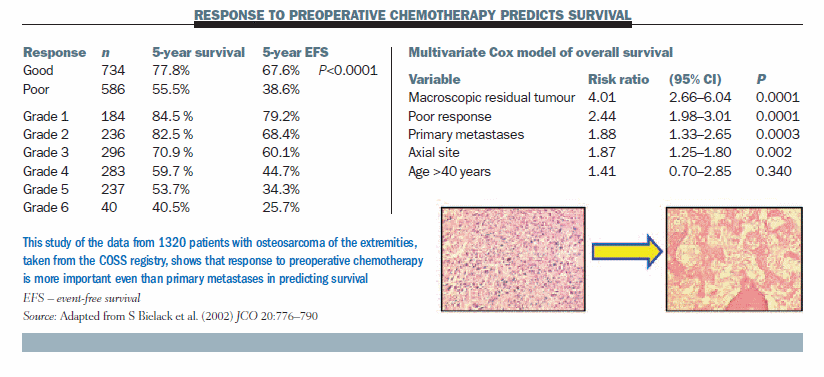
For preoperative chemotherapy, we confirmed that histologic response of the primary tumour is related to five-year survival based on treating 1320 osteosarcomas of the extremities with or without primary metastases. Histologic responses to chemotherapy were graded as either good (>90% destroyed) or poor (<90% destroyed; >10% viable). Results (see table below) showed a significant difference in five-year survival and event-free survival across a six-grade histologic response system used in German- speaking countries, ranging from five-year survival of 84.5% in grade 1 (very good response) to 40.5% in grade 6 (very poor response), with a gradual decline in prognosis along the scale (JCO 2002; 20:776–790). If you put histologic response to preoperative chemotherapy into a multivariate model of overall survival it beats even primary metastases as a prognostic factor, so chemotherapy response is very important.
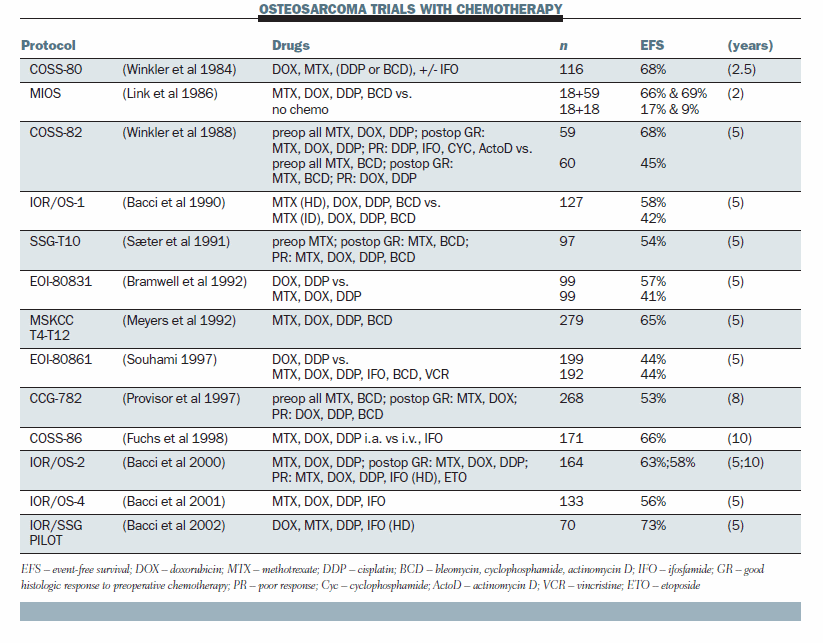
 A summary of osteosarcoma trials from the early 1980s to the late 1990s shows that everybody uses essentially the same drugs. A randomised trial of chemotherapy versus no chemotherapy, the MIOS trial, showed that chemotherapy is efficacious (NEJM 1986; 314:1600–06). Quite a few trials have tried to use modified postoperative chemotherapy in poor responders but none have shown it works.
A summary of osteosarcoma trials from the early 1980s to the late 1990s shows that everybody uses essentially the same drugs. A randomised trial of chemotherapy versus no chemotherapy, the MIOS trial, showed that chemotherapy is efficacious (NEJM 1986; 314:1600–06). Quite a few trials have tried to use modified postoperative chemotherapy in poor responders but none have shown it works.
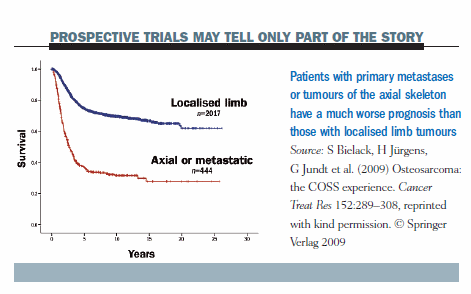
 Prospective trials, which usually include only patients with localised osteosarcomas of the extremities, may tell only part of the story. The prognosis is much poorer for patients who present with primary metastases or tumours of the axial skeleton than for those with localised limb tumours.
Prospective trials, which usually include only patients with localised osteosarcomas of the extremities, may tell only part of the story. The prognosis is much poorer for patients who present with primary metastases or tumours of the axial skeleton than for those with localised limb tumours.
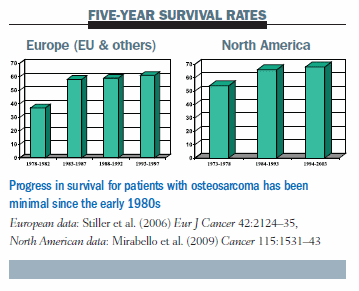
 The bar chart below illustrates the progress in Europe and in North America in the last 20 years in terms of five-year survival: basically, there is none. The survival rates have been stable since 1980, which is not really surprising, as we have been using the same drugs since then.
The bar chart below illustrates the progress in Europe and in North America in the last 20 years in terms of five-year survival: basically, there is none. The survival rates have been stable since 1980, which is not really surprising, as we have been using the same drugs since then.
 The take home messages for osteosarcoma chemotherapy are:
The take home messages for osteosarcoma chemotherapy are:
- Giving chemotherapy is much better than not; multicentre groups can achieve similar results to single centres; and patients with localised extremity osteosarcomas do better than those with other tumours.
- Almost everyone uses preoperative chemotherapy; however, survival outcomes are similar when you operate immediately.
- Poor response to preoperative chemotherapy is a very bad thing. People try to improve results in poor responders by adding drugs and increasing intensity postoperatively, but we do not know whether this works.
- Almost everyone uses the same drugs (two to four of: high-dose methotrexate, doxorubicin, cisplatin, ifosfamide).
- Nothing much has changed over the years in terms of five-year survival.
Chemotherapy dose intensity
A retrospective analysis from Italy suggested that patients who had chemotherapy given at a high dose intensity had a much better prognosis than patients who received less than their allotted amount of chemotherapy over time (Oncol Rep 2001; 8:883–888). There were two other retrospective analyses, one from the European Osteosarcoma Intergroup, which looked at doxorubicin and cisplatin (JCO 2000; 18:4028–37) and one from our group, which looked at methotrexate, doxorubicin, cisplatin and ifosfamide combinations (Pediatr Blood Cancer 2006; 47:42–50). Neither showed that patients who had higher dose intensity chemotherapy had better outcomes.
The question of dose intensity was looked at in a randomised trial by a British/Dutch/Belgian/Danish group. Patients were randomised to conventional chemotherapy (cisplatin plus doxorubicin, two cycles preoperatively and four postoperatively) or an intensified arm where G-CSF was added as support, and dosing intervals were compressed from three to two weeks, which meant that three cycles were given before surgery and three cycles after surgery. The response rate was higher for the compressed arm (50% vs 36%). But five-year progression-free survival rates were identical for the compressed and the conventional arms (41% vs 39%) (JNCI 2007; 99:112–128).
Retrospective and prospective analysis of high-dose chemo-therapy with stem cell rescue – the ultimate dose intensification – did not show improved prognosis, so the take home message is that we are not likely to improve results by dose intensification.
The question we need to ask is whether we can improve outcomes for poor responders, because their long-term survival rates are well below 50%. To look at this we would need to randomise approximately 700 patients, which means that we need about 1400 patients who are willing to be randomised after having received 10 weeks of preoperative chemo, which means we need far more than 2000 patients to go into such a trial. With a disease that occurs in only about two per million people per year, no country can do that by itself, so you need an intergroup collaboration.
That is what we did in the EURAMOS trial, which recruited 2260 patients from 326 institutions in 17 countries from 2005 to 2011. Figures presented by Katja Zils and colleagues at SIOP 2011 (SIOP abstracts, Pediatr Blood Cancer 57:779) showed that complex infrastructures spanning many institutions, countries and even continents, are needed for such a large trial, but that it can be done.
What else can improve prognosis?
Having seen no improvement in prognosis resulting from modifications to chemotherapy we might need to do something else. One possibility may be liposomal muramyl-triphosphate-ethanolamine (L-MTP-PE), a macrophage activator derived from mycobacterial cell wall. Preclinical testing was carried out more than 20 years ago in dogs, and in humans macrophage infiltration into osteosarcoma lung metastases was observed with this drug. It was not clear whether patients survived better, but toxicity was manageable, with mainly fever and chills (JCO 1992; 13:1310–16).
This drug was taken forward into a prospective trial in the US – the INT0133 trial. However, at the time the trial was designed, people thought that asking a question only about MTP would be too simple, so there was a second randomisation of ifosfamide versus no ifosfamide. This was added to cisplatin postoperatively, but in the preoperative phase patients had either ifosfamide or cisplatin. In the end, there were four arms: one with ifosfamide and MTP, one with only ifosfamide, one with only MTP and one with neither. MTP was given 48 times.
The results for 667 patients published in 2005 (JCO 2005; 23:2004–11) showed that the addition of ifosfamide to standard chemotherapy did not enhance event-free survival. The three-year event-free survival rate was 68% for patients receiving MTP but no ifosfamide, compared to 71% for patients who received no ifosfamide and no MTP. Overall, adding ifosfamide to standard chemotherapy did not improve event-free survival. The authors suggested that adding MTP to chemotherapy might improve event-free survival, but there was interaction between the two randomisations to ifosfamide and MTP, precluding definitive statements.
A second publication showed six-year event-free survival of 64% for patients treated with neither ifosfamide nor MTP and 63% for those given additional MTP but still no ifosfamide (JCO 2008; 26:633–638), so there was no difference favouring MTP observed in patients not treated with ifosfamide. Patients seemed to do better with ifosfamide plus MTP (71%) compared to ifosfamide with no MTP (58%). Combining arms showed the six-year event-free survival was 61% for non-MTP arms and 67% for MTP arms, which was not significant. However, overall survival for the combined MTP arms was statistically better than for the combined non-MTP arms (78% vs 70%, P=0.03). The authors said they could not prove interaction, so concluded there was no interaction.
L-MTP-PE (mifamurtide) is now licensed in Europe, but a license was refused in the US because the FDA considered there was not sufficient evidence of a survival advantage. In a letter to the JCO, several leaders of international osteosarcoma groups said they considered it was an interesting agent, but that additional clinical evaluations are required before it can be considered for routine use (JCO 2008; 26:3102–03). I think that the information is not sufficient to use this agent as a part of routine treatment today, but we should continue to study it, preferably in a randomised prospective trial. An international group that met in London in 2010 concluded that an MTP trial should be performed, comparing chemotherapy with and without MTP.
Other options for trials include: optimising chemotherapy schedules; inhaled GM-CSF to enhance immune response to osteosarcoma cells (but this was tried and failed; Clin Cancer Res 2010; 16:4024–30); IGF-1R inhibitors, which showed positive in vitro results, but no positive phase II data so far in osteosarcoma; mTOR antagonists, with (somewhat) positive phase II data in bone tumours (JCO 2012; 30:78–84); bisphosphonates, which are being tried in various trials; and a rank ligand inhibitor, denosumab. Most of these, apart from bisphosphonates, are not yet advanced enough to go into phase III trials.
In summary
The take home messages are:
- Exact staging for osteosarcoma is mandatory.
- Cure requires good surgery and good chemotherapy, which should include several of the four standard agents, which are doxorubicin, cisplatin, methotrexate and ifosfamide. We do not know the value of additional drugs.
- Intergroup collaboration is helpful to get to results and is also feasible, although not easy. For the future, we need biology-driven questions and we must work to ensure that intergroup studies come up with biology
- results that can lead to biology questions for future trials.
Question: What is the current standard of care for osteosarcoma now that the EURAMOS trial is closed? This relates particularly to patients who have a poor histological response to three-drug chemotherapy with cisplatin, doxorubicin and methotrexate. There is always a temptation to give patients different chemotherapy postoperatively if they have had a poor histological response, but would you say that the standard of care should still be three-drug chemotherapy in this subset?
Answer: Yes. I think that whatever well-chosen chemotherapy you give pre- operatively should be the standard for postoperative treatment as well. Nobody has been able to prove that changing chemotherapy by making it more aggressive will ultimately alter the disease course. We will have to wait a while to see whether intensification with ifosfamide and etoposide will result in a higher cure rate.
Question: Is there a role at all for high-dose chemotherapy and stem cell support for osteosarcomas in an inoperable site.
Answer: No!
Question: Regarding MTP, do you think we would be having the same degree of soul searching about its use if it was less expensive?
Answer: Cost is one thing. But there are two other issues that concern me. One is the additional burden for the patient. They will have to go to the hospital 48 additional times for their infusions – this is 48 days of their lives, which is a lot for patients who are living their last days. I would rather they do other things than sit in a hospital being treated with a drug that may not work. The other issue is toxicities, which we need to consider when planning future trials with biologic agents. It will be more difficult to add them to a standard regimen that includes MTP than to one that does not. If we add MTP to the standard regimen we should be quite sure that it is a drug that really benefits the patient, otherwise it will be more difficult to move forward.
The European School of Oncology presents weekly e-grandrounds which offer participants the opportunity to discuss a range of cutting-edge issues, from controversial areas and the latest scientific developments to challenging clinical cases, with leading European experts in the field. One of these is selected for publication in each issue of Cancer World. In this issue, Stefan Bielack, from Stuttgart’s Olgahospital, in Germany, provides an update on recent clinical trials in osteosarcoma and the implications of the findings for future studies. Bruce Morland, from the Birmingham Children’s Hospital in the UK, poses questions arising during the e-grandround live presentation. The presentation was summarised by Susan Mayor.



