Hodgkin’s patients survive for longer but with more serious and lasting damage than almost any other group of cancer patients. Finding ways to address the problems of both current and future survivors can provide valuable lessons for other cancers.
Why should we bother with Hodgkin lymphoma? It accounts for only 1% of cancers, and the probability of cure has improved dramatically over the past 40 years, with 94% of patients now expected to survive. Does it really deserve research funds and the attention of the wider cancer community?
The answer from the Hodgkin’s research community is an emphatic yes. In the 1970s Hodgkin’s became one of the first curable diseases in oncology, and ever since there have been important debates about best treatment and long-term risk/benefit balance that have real relevance to the wider world of cancer.
Hodgkin lymphoma, which most commonly affects young adults, focuses minds on patients’ lives: how to give them decades of life free not only from the effects of cancer, but also from the effects of treatments used to cure them. These issues can only become of wider importance as more and more of the global population experiences cancer as a long-term condition. In the words of a recent editorial by Joseph M Connors in the New England Journal of Medicine, Hodgkin’s is “The Great Teacher”.
A leader in the quest for more acceptable, less toxic, approaches to treating Hodgkin lymphoma is Andreas Engert, chairman of the German Hodgkin Study Group (GHSG) and professor of internal medicine, haematology and oncology at the University Hospital of Cologne. He knows only too well that the problem with Hodgkin’s is not so much how to cure it, but how not to kill with the treatments. Engert was only 14 when his father was diagnosed with Hodgkin lymphoma. He was cured with chemotherapy, but then died 16 years later – when Engert had already embarked on his career in oncology – from cardiovascular problems resulting from treatment.
Such outcomes are not uncommon. Studies indicate that the risk of developing neoplasms after treatment for Hodgkin’s is 22% at 25 years – an 18-fold increased risk compared to the rest of the population. People who have survived Hodgkin lymphoma also have a significantly increased risk of coronary artery disease, valve disease, congestive heart failure, pericardial disease, stroke, arrhythmia and sudden cardiac death.
Since his father’s death, Engert’s career has been defined by searching for alternatives to current chemotherapy and radiotherapy regimens. It has been a quest that has seen him following and then taking up the mantle from Volker Diehl – the man who treated his father and one of the European giants in Hodgkin’s research – and then firmly establishing his centre as a world leader in developing and evaluating new approaches for haematological and solid cancers.
In the process, Engert has sometimes found himself cast as a young radical, determined not to be bound by history, restless to move the agenda forward, and sometimes in profound disagreement with many in the Hodgkin’s community about the right balance between effectiveness and toxicity in treatments.
But today Engert, now assistant director of the Department of Internal Medicine at the University Hospital of Cologne, believes that the long search for less lethal cures may have reached a vital juncture. The days of tortuous debate about which chemotherapy regimens are best may be numbered.
Engert is keen to talk about a new targeted drug – brentuximab vedotin, codenamed SGN-35 – which he says may be about to change everything in Hodgkin lymphoma. Results from trials in the US presented at ASCO last year showed that the drug induced remission in 75% of patients with relapsed or refractory Hodgkin’s, with 35% achieving long-lasting and complete remission.
What is more, because it is an antibody-drug-conjugate – a combination of an antibody and a drug guided safely to its target – the dangers of systemic treatment and radiotherapy are avoided. The drug was registered in the US last year, and is expected to be registered in Europe soon.
“This is the single most effective drug we now have for Hodgkin’s,” says Engert, whose German group has successfully trialled the drug in refractory or relapsed disease. “It’s thrilling for me – and I’m not just saying this because we get money from the pharmaceutical company to conduct clinical trials. It’s thrilling because I worked on linking antibodies with plant toxins in the late 1980s when I was at the Imperial Cancer Research Fund. Trials eventually showed that these immunotoxins were not good enough because they were immunogenic [produced an immune response]. But now, 20 years later, this company has produced a very well-tolerated combination. So that story, for me, has now come full circle.”
The story has come full circle in more ways than one. Six years ago, Volker Diehl – who led the development of the BEACOPP chemotherapy regimen for advanced Hodgkin lymphoma – described the regimen as a “great poison” in an interview with Cancer World. “I would like to have something better,” said Diehl, who had spent decades working on improvements to chemotherapy. “Sometimes I wake up in the night and ask what will happen to my young patients in 10 to 15 years.”
Now Engert, who was co-ordinating editor of the Cochrane Haematological Malignancies Group for 10 years, believes that “something better” may have arrived. What is more, after 30 years when monoclonal antibodies were being developed for other cancers, but there were no new drugs for Hodgkin’s, several new targeted drugs for the disease are now in trials.
“The drug companies are now knocking on our door,” says Engert. “Before, they always said it was too small a market – that since you cure most of these patients anyway, there’s no use looking at the 10–20% who are not cured. They wanted to invest their money in other areas such as lung cancer. We had discussions for many years, with many drug companies. I remember in the 1990s we found a bispecific molecule that was pretty effective and Schering was interested in it, but the guys who had the money did the calculations and said no.
“It has been frustrating, given progress on new targeted drugs for other cancers. My interest has always been on antibodies, since I was a medical student. I remember that when rituximab first came out for non-Hodgkin lymphoma, nobody was interested. They said antibodies are fine for diagnostics, for distinguishing between different tumour types. But treatment? No way. We were one of the first European centres to take rituximab and use it in combination with chemotherapy, and for diseases like chronic neutrophilic leukaemia.”
“They said antibodies are fine for distinguishing between tumour types. But treatment? No Way”
Born 1959 in Braunschweig, Germany, Engert rebelled against his father’s wishes that he should become a banker, and decided to study medicine. When he was a medical student, his father kept urging him to go and see Volker Diehl in Hanover, the man who had cured his Hodgkin’s with chemotherapy. “I said no,” says Engert, with a smile. “Whatever you say, I do exactly the opposite – that’s what many young men do I guess.” As a medical student, he had initially focused on psychiatry and psychosomatics, then decided it wasn’t for him. He knew he didn’t want to go into surgery, and he also knew that it was internal medicine – immunology, nephrology and oncology – that he found most interesting. So he changed his mind about Diehl.
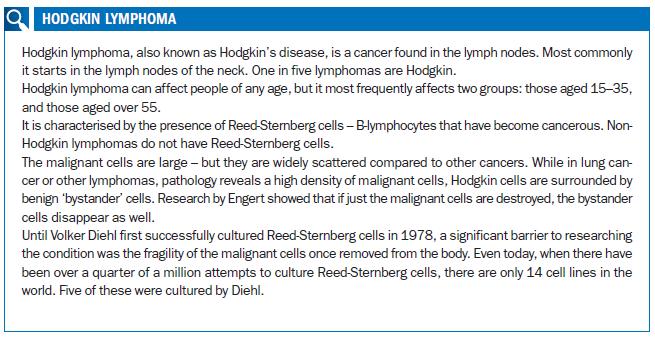
“I realised that he was a really interesting doctor. I worked for him, wrote my thesis with him, and then followed him. My father and Volker Diehl were certainly very influential on the direction I took.” Indeed Diehl – who famously cultured notoriously fragile Hodgkin cell lines for the first time, opening up new worlds of research possibilities – seems to have exerted an almost gravitational force on the trajectory of Engert’s career.
When Diehl moved from Hanover to the University of Cologne, to become director of the Department of Medicine and chairman of the German Hodgkin Study Group, Engert took up the offer to come with him – despite other offers to work in oncology, nephrology and immunology in Hanover. He worked with Diehl for two years, but when offered a six year contract, he again rebelled.
“I said I didn’t want it – I want to go into research.” That was when he spent two and a half years researching antibody-based immunotherapy for patients with malignant lymphoma under professor Philip Thorpe at the Imperial Cancer Research Fund in London: “It was a great time, to learn all the techniques and have time for science only.” But then Thorpe went to Dallas. Engert didn’t want to follow him to America but he did want to continue work on trialling the promising immunotoxin drugs he had helped develop in London. So in 1991 he decided to rejoin Diehl in Cologne, where as head of the laboratory of immunotherapy he could conduct phase I and II clinical trials on the new drugs – which ultimately proved disappointing.
“It was a great time, to learn all the techniques and have time for science only”
Engert has remained there ever since, becoming deputy medical director in 1999, and taking over from Diehl as chairman of the GHSG in 2007. He fell in love with Cologne, he says. A sense of the city’s scientific heritage hangs around the vast atrium of the University Hospital where he works, with photographs and memorials to the likes of medieval experimenter Albertus Magnus and heart radiography pioneer Friedrich Moritz.
As for the shadow of his predecessor, Engert has a pragmatic view – respectful, but clear that if things are to progress, past achievements have to be viewed as inadequate. There is little room for sentiment in medicine.
“I had had my own interest in immunotherapy for a long time and brought a lot of new treatment aspects into the group,” he says. “I supported Volker and worked with him, and when he left the hospital and I took over, there were many things I maintained – but it was good for me to have more freedom to run things as I would have loved to before.”
“Over these years the knowledge and experience of this group has broadened substantially,” he says. “Volker’s work allowed us to become one of the leading groups on this disease worldwide, and we really appreciate that. But things have evolved and adapted to modern aspects of treatment and how studies have to be done.” The GHSG today recruits patients from more than 400 centres in five European countries.
The increasing influence of the group has led it into intense exchanges with other groups – debates about best treatment that Engert believes are extremely important for clinical progress, and which he admits to finding enjoyable. “It would be boring if everyone had the same opinion and there was no more development,” he says.
One of the most important has been about what should be the standard chemotherapy regimen for advanced Hodgkin lymphoma. The debate, conducted over the past five years on the pages of the most high-powered professional journals, has seen the German group pitted against groups in America, the UK and Italy.
It started in 2004 when Engert and Diehl presented the findings of a major study which showed that the German group’s BEACOPP regimen was 20% better at tumour control and 11% better for overall survival than current standard chemotherapy regimens revolving around ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine). What is more, the benefits became clearer the longer patients were followed up.
But American study groups took issue: BEACOPP was too toxic for standard use, they said. Engert acknowledges they had a point: it is more toxic than ABVD. But what these other international groups – the “anti-BEACOPP alliance” as Engert calls them – have failed to acknowledge, he claims, is that it is also much more effective.
Another study, published in the New England Journal of Medicine last July by Viviani and colleagues at the Istituto Nazionale Tumori in Milan, indicated that BEACOPP was 12% better at tumour control than AVBD. But, according to Engert, the article and accompanying editorial instead concentrated on the fact that overall survival with BEACOPP was no better than with the less toxic ABVD – “which is not surprising given the small number of patients in the study.”
“They were basically saying this study showed that ABVD was as good as BEACOPP, which we think is unfair. We always said that BEACOPP is more toxic than ABVD. But we all have to stick to facts and not overshoot in our attempts to disprove one another.”
The Istituto Nazionale Tumori meanwhile is emphatic that “we have not hidden or confused the message.”
“We all have to stick to facts and not overshoot in our attempts to disprove one another”
The debate is moving on again, with the German group providing evidence in a Lancet article this May that less toxic doses of BEACOPP are equally effective as larger doses. Engert is all too aware of the awkward balance between risk and benefit, acknowledging that sometimes a high risk is necessary whilst doing everything possible to minimise it.
What such debates reveal to Engert is the importance of following the patient’s story for as long as possible after treatment, not jumping to conclusions after short-term studies. “The stories of terrible side-effects aren’t over yet,” he says. “Hodgkin’s is a great teacher because you can follow patients carefully through to the end – the consequences of their treatment may not be visible for 20 or 30 years. We are certainly keen to get rid of chemotherapy and radiotherapy. However, this certainly cannot be done at the price of a very high relapse rate.”
It is natural enough that patients, oncologists and indeed drug companies should want to concentrate on cure. But people who have been treated for Hodgkin’s form the biggest cancer survivor group in Western countries, with 70–80,000 in Germany alone, so that researchers and clinicians are able to look far beyond remission. Hodgkin’s, says Engert, can serve as a ‘model cancer’, providing answers not just on side-effects, but also on how to support patients long-term through enduring problems such as infertility and fatigue.
This human element of the oncologist’s role is important to Engert, who has always tried to balance laboratory work with clinical work. One of the reasons he went into oncology was that it seemed less mechanistic, more based on long-term relationships with people, than specialties such as cardiology. The relationship is not always a simple one, he acknowledges. Patients tend to focus solely on cure at the start of their journey, and oncologists have to be careful to counsel them about side-effects such as infertility, hair loss and fatigue that will become of major importance to them later. The complexity of some decision-making requires meaningful collaboration.
“Pretty early on I realised that cancer patients give back a lot,” says Engert. “They realise they have to work with you, and want to co-operate. You see them develop in this respect, whereas in other areas such as cardiology, people are hardly off the intensive care unit after heart attack and they’re off to work again, and there’s no long-term working relationship with the doctor.”
“Pretty early on I realised that cancer patients give back a lot, they want to co-operate”
What about Engert’s human side? Given the motivating role played by his father’s experiences with cancer, how much does his own young family know about his work and what he is trying to do? Aged 53, married to an artist, he has four children aged between 10 and 17, and their photographs cover one wall of his office. He tries to devote as much time as possible to home life, but the demands of work and international conferences don’t make this easy and sometimes he brings his children into work or takes them to conferences with him.
Sometimes he has his doubts about how much of his work his children really understand, but he does know that, among friends and family of the children’s friends, there have been several with cancer who have asked Engert for advice. The children know about this and discuss it – there is no taboo around cancer. And Engert admits that he would be “honoured” if any of them decided to follow him into medicine. Perhaps that story too will come full circle.
If it does, the challenges his children face may be very different than Engert’s. “I think this new drug, and others to come after it, will change treatment for Hodgkin’s fundamentally,” he says. Already well-tested in relapsed patients, brentuximab vedotin is now undergoing trials as a maintenance treatment, with the aim of reducing the number of relapses. Engert believes that in two years, therapy for relapsed patients will have changed substantially, and within a decade chemotherapy and radiotherapy will no longer be mainstays of treatment.
Does that mean everything is rosy? Not quite. For those who specialise in Hodgkin’s, there is always the nagging worry about the unforeseen future – the worries about treatment side-effects that gave Volker Diehl sleepless nights. Engert too knows the risks that can come with new treatments. In 2006 he advised the biotech company Te Genero not to test its antibody TGN1412 on healthy volunteers – advice the company rejected. As a result, 10 volunteers in London suffered near-fatal side effects. “I told them it was nonsense to try this drug on healthy volunteers in this type of trial – you can’t do this. But they did.”
It all goes to show that there is no room for complacency in treatment development. “Drugs aren’t like water,” says Engert. “If we had a shiny new side-effect-free drug, and then a year after its introduction patients started dying from secondary cancer then, yes, I too would start having night sweats and nightmares.”
For the moment, he is cautiously confident. But he knows only too well that the greatest arbiter of treatment success is time.