Engaging the body’s own immune system in controlling cancer has long been an aspiration for cancer researchers and clinicians. This overview looks at progress towards that goal, and how it is changing the paradigm of cancer treatment.
This grandround was first presented by Aleksandra Filipovic, from the Department of Surgery and Cancer, division of cancer, Imperial College, London, as a live webcast for the European School of Oncology. It is edited by Susan Mayor. The webcast of this and other educational sessions can be accessed at e-eso.net.
Death rates due to cancer fell by only 8% between 1950 and 2012. Other diseases saw death rates fall markedly over the same period: by 67% in the case of heart disease, 77% for cerebrovascular diseases and 66% for pneumonia and influenza.
Why was the reduction in cancer death rates so much lower than in other diseases? Recent developments suggest that our understanding of cancer was oversimplified. However, understanding of tumour immunology has increased dramatically over recent years, which has led to the development of immuno-oncology agents that have created a paradigm shift, addressing areas not covered by previous treatment options.
The immune system and cancer
At a simplified level, a tumour can be considered as a mass of tissue growing in an organ where it is not supposed to be. Tumours contain cancer cells, blood vessels that supply nutrients to the cancer, cells involved in the inflammatory response, and connective tissue cells, in addition to cells from the immune system. Tumour cells initially grow in a primary organ before spreading through lymph nodes or blood vessels, or both, to distant sites. This is a complex process requiring cancer cells to leave the primary tumour, enter the lymphatic system or blood vessels, and travel around the body before exiting and establishing themselves in a secondary site. Each step of this metastatic process involves a significant contribution from cells of the immune system, particularly T cells.
Immuno-oncology agents
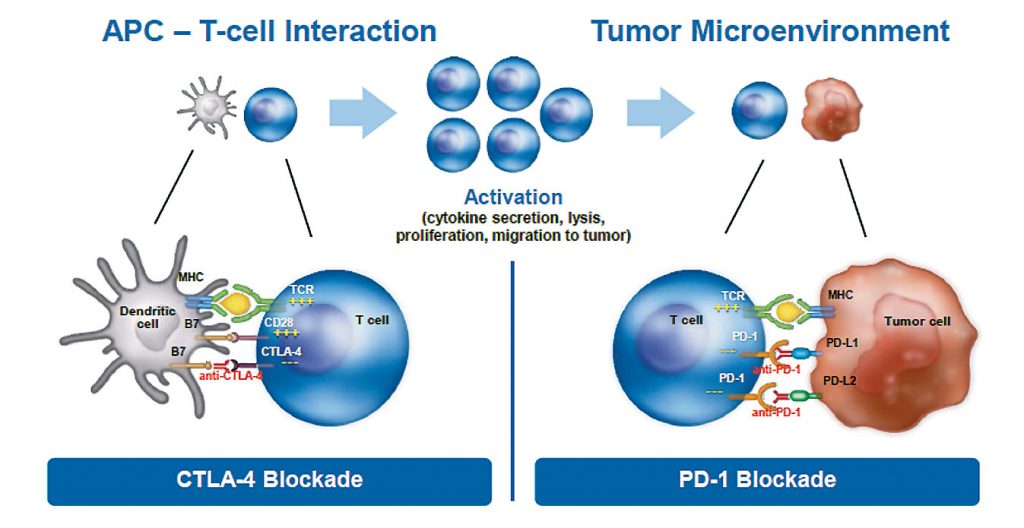
The first immuno-oncology agent to be approved for treating solid tumours was the anti-CTLA-4 antibody ipilimumab, followed by anti-PD-1 antibodies, including nivolumab and pembrolizumab. They have very distinct mechanisms of action, blocking the inhibition that cancer cells impose on the immune system.
Cancer immunity cycle
The cancer immunity cycle provides the framework for how we can manipulate the immune system to attack and destroy tumour cells (click to enlarge image).
Source: DS Chen and I Mellman (2013) Oncology Meets Immunology:
The Cancer-Immunity Cycle. Immunity 39:1-10.
Republished with permission from Elsevier.
Oncologists and researchers have been trying for decades to activate or boost the immune system in targeting cancer. Until recently we failed, because we did not understand the mechanism underlying the intricate interaction between tumour cells and cells of the immune system. We now understand that a cancer cell interacts with a T cell by direct contact through receptors such as CTLA-4, PD-1 or PD-L1, causing inhibition of the immune system.
These new immuno-oncology drugs block the interaction between cancer cells and cells from the immune system, effectively creating a firewall that prevents inhibition from occurring. These drugs do not activate the immune system but, instead, they inhibit the inhibition that cancer cells impose on immune cells.
What does immuno-oncology add to treatment outcomes?
Chemotherapy prolongs survival to a certain extent, and the new generation of targeted agents contribute to further prolongation of survival. However, most cancer patients inevitably die of their disease. Immunotherapy lifts the survival curve, with anywhere between 5% and 30% of patients under the curve who survive and continue to live with their disease, even if immunotherapy is introduced late in their disease, at an advanced or metastatic stage.
Pivotal clinical trials with immunotherapy agents
Ipilimumab was approved on the basis of a phase III trial that showed important survival gains for patients with metastatic malignant melanoma, with 24% alive at two years compared to only 14% with the peptide vaccine gp100 (NEJM 2010, 363: 711–23).
The CheckMate 057 Trial, which led to the approval of nivolumab in metastatic lung cancer, was stopped early, with an interim analysis showing that nivolumab prolonged overall survival by three months (NEJM 2015, 373:1627–39).
A trial leading to the approval of pembrolizumab in lung cancer showed overall survival was significantly longer than with docetaxel (median 14.9 vs 8.2 months) (Lancet 2016, 387:1540–50).
The CheckMate 025 study of nivolumab in renal cell carcinoma showed prolongation of overall survival to 25 months compared to 19.6 months with everolimus (NEJM 2015, 373:1803–13). Immune checkpoint inhibitors are now approved for melanoma, lung cancer, renal cell carcinoma, and squamous cell head and neck cancer, and are being used worldwide.
The survival curves that led to approval of ipilimumab
Ipilimumab, the first immune checkpoint inhibitor to receive approval, showed a significant improvement in survival for patients with malignant melanoma, with the characteristic tail showing a proportion of patients derive long term benefit (click to enlarge image).
Source: Adapted from FS Hodi et al. (2010) Improved Survival with Ipilimumab
in Patients with Metastatic Melanoma, NEJM 363:711–723. © 2010
Massachusetts Medical Society. Reprinted with permission
Unique features of immunotherapy
Side effects
Immunotherapy delivers survival benefits for patients with many types of solid tumours, but it is important to consider the side effects of these agents. Fatigue is one of the commonest side effects, affect-ing around one-quarter of patients treated with immunotherapy. Grade 3–4 adverse events are uncommon, typically affecting fewer than one in ten patients.
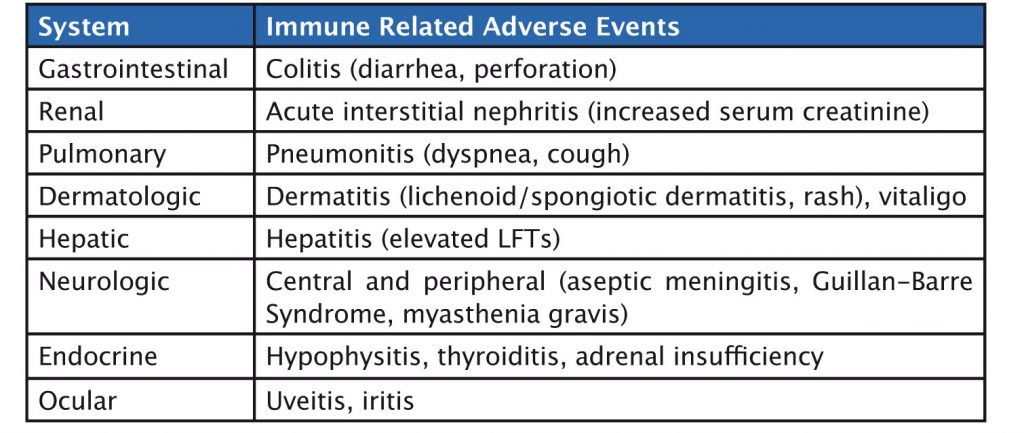
Immunotherapy agents have a specific set of immune-related side effects (see table). Gastrointestinal side effects such as colitis can occur, and, in rare cases, patients have gastrointestinal perforation. Liver-related side effects include hepatitis, while pulmonary adverse events include pneumonitis. Skin, neurologic and endocrine side effects can also occur. Side effects should be graded and treated with corticosteroids until they reduce to grade 1, or resolve, when immunotherapy can be restarted.
Treatment-related adverse events
Fatigue is the most common adverse event (24%)
Grade 3–4 adverse events are uncommon (6-12%)
Liver side effects can be detected by an increase in liver enzymes, and skin side effects by clinical examination. It is also important to monitor thyroid function and adrenal function, and to be aware of clinical symptoms such as cough, which may occur in pneumonitis, or diarrhoea in colitis.
Pseudoprogression
Pseudoprogression is a unique phenomenon that occurs in 7–10% of patients treated with checkpoint inhibitors, and which stems from their mechanism of action. The figure overleaf shows this in scans for two lung cancer patients treated with PD-1 inhibitors. Two months after starting treatment, lesions increased in size compared to the pre-treatment scan, but at four months there was complete response.
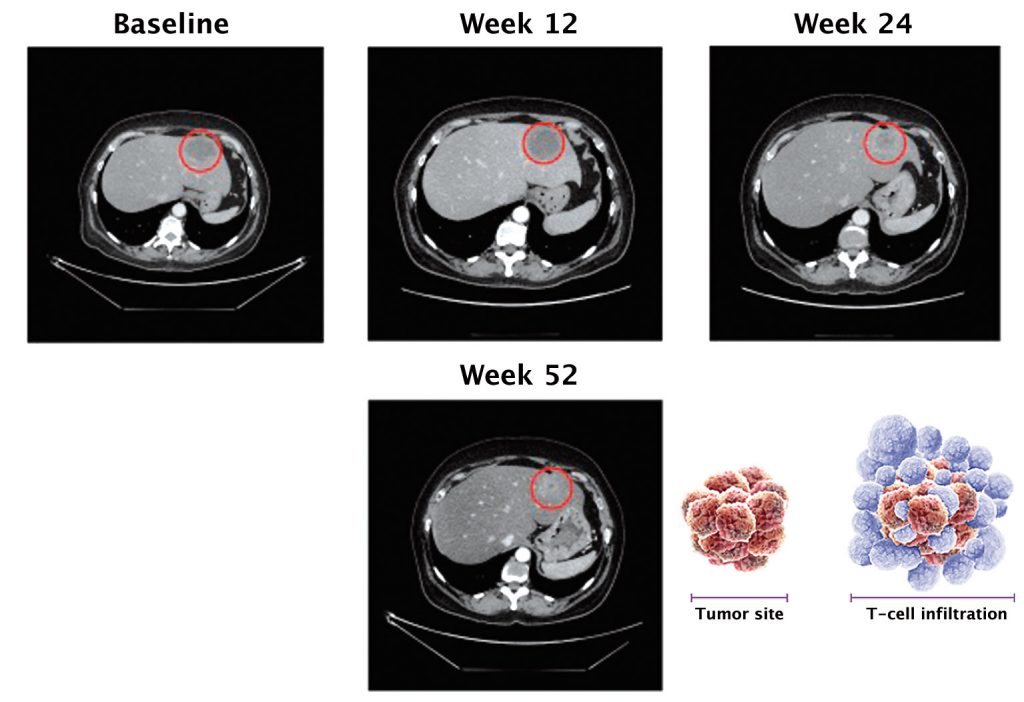
Source: FS Hodi et al. (2016) JCO 34:1510-7, reprinted with permission from the American Society of Clinical Oncology, ©ASCO. All Rights Reserved
If a patient is treated with chemotherapy, it hits the cancer cell head on, and very shortly after starting treatment we would expect to see tumour shrinkage or complete response. However, the whole concept with immunotherapy is to engage the T cells, which gather around tumour cells and elicit cell killing. When T cells reach tumour cells they secrete cytotoxic mediators, which then act to kill the tumour cells.
Question: Are there any other imaging methods that could better detect pseudoprogression?
Answer: At the moment we don’t have any particular imaging methodology that can conclusively identify pseudoprogression. However, these imaging modalities are being developed using a specific type of contrast to detect T cells expressing PD-1 or PD-L1 that have infiltrated a tumour.
Current scanning techniques show only the boundary of the tumour mass, and cannot distinguish where the tumour ends and T cells begin, but we hope to have better techniques within the next few years.
For now, radiologists are starting to better understand what pseudoprogression looks like with current scanning modalities, and it is obviously important for them to be aware that a patient is being treated with immunotherapy.
The timeline is also important: pseudoprogression occurs shortly after starting treatment, but an increase in tumour size at six months, for example, is more likely to be disease progression, so using common clinical sense in these circumstances is very important too.
Question: What proportion of patients experience pseudoprogression?
Answer: Up to 10% of patients experience pseudoprogression, but I think figures in the future will show that the proportion is actually lower than this – that’s why we say “up to 10% max”.
Identifying predictors of response
An important aspect of using immunotherapy agents is selecting the right patients in whom to use them. These are costly drugs, and they are not without side effects –although these are less frequent than with chemotherapy or targeted agents – so we should be choosing the right population to treat.
Identifying predictors of response
Expression of PD-L1 is being investigated as a potential predictive biomarker of response to PD1 and PD-L1 inhibitors. A variety of immunohistochemistry assays are used to measure expression levels, with a variety of cut-off points to indicate positivity (click to enlarge image).
IC – tumour-infiltrating immune cells, TC – tumour cells
Source: Adapted from C Grigg and NA Rizvi (2016) J Immunother Cancer 4:48, reprinted under creative commons licence http://creativecommons.org/licenses/by/4.0/
One of the main indicators being investigated as a biomarker for PD-1 and PD-L1 inhibitors is expression of PD-L1 by tumour cells and also by immune cells infiltrating a tumour. The degree of expression varies considerably across different types of solid tumour (as shown in the table opposite).
Checkpoint inhibitors in development
Many types of checkpoint inhibitor are in development for a wide range of cancers.
CTLA-4 inhibitors: Ipilimumab is approved for metastatic melanoma, and is being trialled for both non-small-cell lung cancer (NSCLC) and small-cell lung cancer; tremelimumab is in development for NSCLC.
PD-1 inhibitors: Nivolumab is approved for NSCLC in Europe and the USA, while pembrolizumab is approved for PD-L1-positive NSCLC in the USA.
PD-L1 inhibitors: Several agents are in pipeline development, including atezolizumab, durvalumab and avelumab.
These agents and other comparators are in clinical development for a large number of other solid tumours.
Anti-PD-1 and anti-PD-L1 agents have different antibody isotypes, either IgG1 or IgG4. This has an impact on how these agents work – whether they engage only cells from the immune system or also elicit involvement of complement- or antibody-dependent cytotoxic cell killing.
Sequencing of immunotherapy
There is a lot of discussion as to whether there is an optimal sequence for using immunotherapy agents. Do we give chemotherapy and then immunotherapy, and can we then go back to chemotherapy? The answer to the latter question is ‘yes’, with several large clinical trials showing that anywhere from one-third (NEJM 2015, 373:123–35) to one-half of patients (NEJM 2015, 373:1627–39) can safely go back to receiving standard of care chemotherapy after immunotherapy.
The anti-CTLA-4 and anti-PD-1 agents that have been approved so far have found their place in the treatment of advanced and metastatic disease, and have gained approval after being tested in second- or third-line treatment. The first-line treatment for metastatic disease is typically standard of care – most likely chemotherapy – and immunotherapy is used when that fails.
Now that we have seen such impressive efficacy with immuno-therapy in later lines of treatment, there is a lot of interest to see how effective and safe these agents are when used first line or combined with chemotherapy from the start.
The CheckMate 012 study is investigating this question in advanced NSCLC, combining nivolumab with different regimens of chemotherapy (gemcitabine, cisplatin, paclitaxel, carboplatin) (CMSTO 2014, Abstract #3). Initial results suggest that the best res-ponse rates are seen with a combination of nivolumab (5 mg/kg) with paclitaxel/carboplatin.
There is a wide range of ongoing clinical phase III trials, including trials of pembrolizumab (Keynote 042) and nivolumab (CheckMate 026) versus chemotherapy of investigator’s choice, as first-line treatment for patients with advanced NSCLC. The AstraZeneca MYSTIC trial is investigating durvalumab (anti-PD-L1) in combination with tremilimumab (anti-CTLA-4) versus durvalumab alone versus chemotherapy in first-line NSCLC.
In addition, there is interest in combining two immunotherapy agents as first-line treatment, for example an anti-PD-L1 agent with an anti-CTLA-4 agent. The MYSTIC trial is combining the anti-PD-L1 agent durvalumab with an anti-CTLA-4 agent tremelimumab (both from AstraZeneca) in first-line treatment of lung cancer, and will report later this year. This may be the first combination of two checkpoint inhibitors in first-line lung cancer treatment.
A similar combination – nivolumab plus ipilimumab (both Bristol-Myers Squibb) – is in a phase I trial (Check-Mate 012). Dosing is important because combining two agents is likely to increase toxicity.
Other trials are investigating the relationship between biomarkers and outcome, such as the KEYNOTE-010 trial investigating levels of PD-L1 expression and outcome with pembrolizumab versus docetaxel in patients with previously treated PD-L1-positive NSCLC.
Following on from the success of checkpoint inhibitors in cancers such as melanoma and NSCLC – both squamous and adenocarcinoma – these agents are now showing signs of efficacy in very difficult-to-treat cancers, such as small-cell lung cancer (SCLC), which represents an important area of unmet medical need.
CheckMate 032, a phase I/II multicentre, multi-arm, open-label trial presented at the 2016 congress of the American Society of Clinical Oncology (ASCO), showed objective response rates in up to 23% of patients with recurrent SCLC, with the anti-PD-L1 agent nivolumab plus the anti-CTLA-4 agent ipilimumab (Lancet Oncol 2016, 17: 883–95), and the combination is being investigated in further trials.
These agents are also being tested in other solid tumours that are not easy to treat and have shown important responses.
Treatment-related adverse events
Tumours with high mutational loads seem to respond particularly well to immunotherapy, and tend to be highly resistant to traditional treatments (click to enlarge image).
Source: LB Alexandrov et al. (2013) Signatures
of mutational processes in human cancer. Nature 2013; 500:415.
Reprinted with permission from Macmillan Publishers Ltd
A study with nivolumab achieved a response rate of 65% in patients with relapsed or progressing Hodgkin lymphoma (www.fda.gov), and a multicentre, non-randomised phase Ib trial in heavily pretreated triple-negative breast cancer showed an 18.5% response rate to pembrolizumab, which is not seen in this setting with chemotherapy (JCO 2016, 34:2460–67).
In previously treated advanced pleural mesothelioma, pembrolizumab showed a disease control rate of 76% in a phase IB trial of 25 people (AACR 2015, abstract #CT103). Excellent response rates have also been seen in gastric cancer and colorectal cancer.
Assessing tumour suitability for immunotherapy
How do we select tumours that may be suitable for treatment with immunotherapy? Mutational rate varies across different types of cancer, with a higher mutational burden in SCLC, NSCLC and melanoma (see figure). Tumours with the highest mutational load have historically been the most difficult to treat, and had the poorest response to standard of care treatments, but are showing good response to immunotherapy agents. Tumours with a lot of mutations are thought to produce more neoantigens. Once the immune system is engaged by the use of checkpoint inhibitors, a tumour producing more neoantigens will be more readily detected as foreign tissue to be destroyed.
A variety of tests can be used to assess mutational burden in tumours in the clinic. These include sequencing, but a simpler test that measures mismatch repair deficiency – a DNA repair process in tumour cells – can also be used. Colorectal cancer tumours harbouring mismatch repair deficiency have shown impressive response rates (62%) to immunotherapy, and high rates of disease control (92%), while mismatch repair proficient colorectal cancers show much lower disease control rates (16%) (JCO 2015, 33 Suppl: Abstract #LBA100). These tests are now being used in other tumours, including pancreatic, gastric and other gastrointestinal cancers.
The scope and rate of approval of immune checkpoint inhibitors in different types of cancer, including melanoma, lung cancer, renal cell carcinoma and Hodgkin lymphoma, and the wealth of clinical trials underway – not only in metastatic and advanced disease but also in the adjuvant setting – suggest there may be a significant increase in their use over the next 10 years. Trials are also being carried out with combinations of immunotherapy agents plus chemotherapy, radiation therapy, targeted agents or with other immunotherapy agents.
I think we are looking at a scenario where immunotherapy could become the backbone of cancer treatment, and other treatment options will be used in a dynamic way around this. Instead of waiting until a patient has metastatic disease, in the future we may be using immunotherapy as first-line treatment and perhaps even in the adjuvant setting.
In summary
Immunotherapy has delivered a paradigm shift in the treatment of cancers so that in the future many patients will live with their disease rather than dying from it. We need to learn how best to use these agents, including defining the best combinations, the patient populations in which to use them and the optimal treatment duration. As these drugs are not cheap, we must also consider how to fund these treatments as they become standard of care.