Suboptimal studies had established preoperative chemoradiation as the preferred strategy in the management of localised oesophageal cancer (LEC) and gastro-oesophageal cancer. The recent CROSS trial has now demonstrated considerable benefit from preoperative chemoradiation over surgery alone in patients with LEC. But are these results only reinforcing advocates of the preoperative chemoradiation strategy?
This article was first published in Nature Reviews Clinical Oncology vol. 9 no. 9, and is published with permission. © 2012 Nature Publishing Group. doi:10.1038/nrclinonc.2012.122
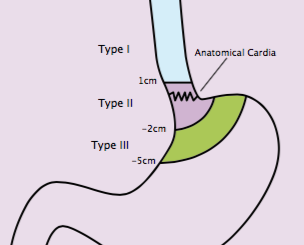
The incidence of gastro-oeso-phageal adenocarcinoma has been rising for the past three decades, possibly owing to the dramatic increase in the BMI of adults in many societies, which has led to chronic gastro-oesophageal reflux disease and Barrett’s oesophagus.[1–3] Squamous-cell carcinoma remains the most frequent histology in the endemic areas of the world, whereas adenocarcinoma is now the most common form of gastro-oesophageal cancer in the USA and parts of the Western World.[4] Historically, the management of localised oesophageal cancer (LEC) has been a source of intense debate. Complexities in the clinical decision-making for patients with LEC include the location of the primary tumour, histological subtype and tumour grade, clinical T and N stages, length of the tumour, ability of the patient to withstand surgery, and prevailing practice patterns. There may be unity in how to manage early-stage disease endoscopically (for example, stage Tis [carcinoma in situ] and T1a tumours), but opinion is divided in terms of how to manage thoracic T2–T3 tumours with any N stage or T1N+ tumours. In patients who can withstand surgery, thoracic LEC is best managed by multimodal therapy – preoperative chemotherapy or preoperative chemoradiation – because the five-year survival rates from primary surgery are dismal,[5] although high-volume centres have reduced surgical mortality considerably.[6] Multidisciplinary evaluation before starting any therapy is encouraged; however, it is not the norm in many countries (such as China and India, where most patients undergo surgery directly). In fact, preoperative chemotherapy for thoracic LEC is largely abandoned in North America but remains popular in many European countries.
Regarding the preoperative chemo-therapy strategy, a North American randomised trial of this approach reported no benefit,[7] and a larger British trial from the Medical Research Council demonstrated only marginal benefit.[8] Preoperative chemoradiation, however, may be establishing itself as the strongest contender among all strategies.
Results from the CROSS trial[9] conducted in Europe, have demonstrated considerable benefit from preoperative chemoradiation over surgery alone in selected patients with LEC. Yet the CROSS study may be reinforcing only the subscribers of the preoperative chemoradiation strategy and may not convert the proponents of preoperative chemotherapy or primary surgery. We feel that the report published by van Hagen et al.,[9] which represents the largest trial of its kind, may be transformative, although, we have our doubts about its impact on global approaches to LEC. Van Hagen et al.[9] randomly assigned 368 LEC patients with histologically confirmed squamous-cell carcinoma or adenocarcinoma (tumour stage T1N1 or T2–T3 with any N) to receive preoperative chemoradiation with paclitaxel and carboplatin in combination with 41.4 Gy of 3-D conformal radiation technique in 23 fractions given five days per week (n=180) or surgery alone (n=188). Although the tumours were well staged at baseline (even if no PET was carried out), patients were still selected according to age (18–75 years), weight loss (≤10%), and tumour not exceeding 8 cm in length or 5 cm in width. With a median follow up of 45.4 months, the median overall survival for the group receiving preoperative chemoradiation was 49.4 months versus 24 months for the surgery-only group (HR 0.67, 95%CI 0.495–0.871; P=0.003). The five-year overall survival rate was 47% versus 34%, favouring the chemoradiation group. This benefit was observed in both histological subgroups studied; however, the effect in the adenocarcinoma group (the largest cohort of the two histological subtypes: 275 patients vs 46) was marginal (P=0.049). Chemoradiation did not lead to excessive toxicity. Other benefits from preoperative chemoradiation included a higher rate of R0 resection and, as expected, a higher rate of pathological complete response in the surgical specimen. Data also supported the use of a moderate radiation dose of 41.4 Gy.
“Yet, the CROSS study may be reinforcing only the subscribers of preoperative chemoradiation”
The CROSS trial is a well conceived and well-executed study that establishes level 1 evidence for preoperative chemoradiation for thoracic LEC and gastro-oesophageal cancers stage T1N1 or T2–T3 with any N stage. However, we are doubtful that these results will help establish a uniform global strategy for this group of LEC patients. This is in part because van Hagen et al.[9] are not optimistic about the preoperative chemoradiation strategy for patients with thoracic LEC and leave the door open to other options (even though the evidence of the benefit of other options is dubious). What will it take for us to have one global strategy for this group of patients with LEC? The answer to this quandary is unclear. However, those oncologists who prefer preoperative chemo-radiation are on firm ground because of the CROSS trial results, and those who believe in surgery first or preoperative chemotherapy have little to base this on. We recommend preoperative chemoradiation as the preferred approach for the group of patients studied in the CROSS trial. We do not endorse surgery first or preoperative chemotherapy under normal clinical conditions, in which there is no contraindication to radiation. It is time to CROSS over.
Significant challenges remain when dealing with a difficult disease such as LEC. We must develop strategies that are appropriate for each histological subtype, for each anatomic location of LEC, and for each stage group of LEC. We must exploit the information provided by better imaging, such as PET. We must develop imaging methods that provide highly specific information – those that can image proliferation, apoptosis, hypoxia, receptor proteins, etc. before and after chemoradiation. Circulating tumour cells, mRNA, DNA, and miRNA can also increase our understanding of the aggressiveness of the cancer. We must carry out in-depth analyses of the molecular biology underlying oesophageal and gastro-oesophageal junction cancer and the genetic profile of the patients. We must focus more on methods to enable the immune system to recognise oesophageal and gastro-oesophageal cancer. Finally, we must identify patients who do not require oesophagectomy because their cancer is highly sensitive to chemoradiation.
We feel this can be achieved through establishing validated biomarker signatures and/or sophisticated imaging studies. We must, therefore, strive for oesophageal and gastro-oesophageal junction preservation and customisation of therapy. These are real challenges to deal with while we are debating our therapeutic preferences. These preferences are usually empiric in nature and help only a few patients, while we subject each one of our patients to the toxicity of preoperative therapy and significant life-altering consequences of oesophagectomy.[10] We would be remiss not to mention that we must also identify adults who are at high risk for developing oesophageal cancer, and detect and treat their cancers early. We may have the roadmap for making progress against LEC, but we seem to be walking in multiple directions. It is time to collaborate.
References
1. H Hampel, NS Abraham, HB El-Serag. (2005) Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 143:199–211
2. LM Brown, SS Devesa, WH Chow. (2008) Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. JNCI 100:1184–87
3. LM Brown et al. (1995) Adenocarcinoma of the esophagus: role of obesity and diet. JNCI 87:104–109
4. Answers™. (2012) Esophageal cancer [online] http://www.answers.com/topic/esophageal-cancer
5. TW Rice et al. (2009) Worldwide esophageal cancer collaboration. Dis Esophagus 22:1–8
6. JD Birkmeyer, Y Sun, SL Wong et al. (2007) Hospital volume and late survival after cancer surgery. Ann Surg 245:777–783
7. DP Kelsen et al. (1998) Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. NEJM. 339:1979–84
8. WH Allum, SP Stenning, J Bancewicz et al. (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. JCO 27:5062–67
9. P van Hagen et al. (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. NEJM 366:2074–84
10. P Viklund et al. (2006) Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg 243:204–211
Author affiliations
Mariela Blum and Jaffer Ajani, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas