What does our growing understanding of the molecular subtypes of gastric and oesophageal cancers, and the results of recent trials testing multimodal therapies, mean for the way we classify, diagnose and treat these tumours? Jonas Feilchenfeldt presents key points from the 2018 St Gallen GI Cancer Conference.
This grandround was first presented by Jonas Feilchenfeldt, from the National Center for Cancer Care and Research, Doha, Qatar, as a live webcast for the European School of Oncology. Marco Siano, from the Cantonal Hospital, St Gallen, Switzerland, posed questions raised during the e-grandround presentation. It was edited by Susan Mayor. The webcast of this and other e-sessions can be accessed at e-eso.net
The St Gallen International GI Cancer Conference has met every two years since 2012 to review the latest research and understanding in primary gastrointestinal (GI) cancers, and issue consensus recommendations for treatment and care.
The fourth such conference, which met in 2018, focused on early oesophageal and gastric cancers. It started by addressing the question of whether there is a need to differentiate histologically between oesophageal and gastric cancer.
Reviewing the evidence available to try to answer this provocative question, Christoph Roecken (Kiel, Germany) explained that there are several classifications for gastric cancer, with the Laurén classification most commonly used in Europe. More insights were gained with the publication of the new classification of gastric cancer in 2014 from The Cancer Genome Atlas (TCGA) Network study (Nature 2014, 513:202–9). This analysis of samples of gastric cancers from different centres around the world proposed a new classification based on four subcategories:
- Epstein-Barr virus (EBV) infection – characterised by frequent PIK3CA mutation and PD-L1/2 overexpression. This variant is more prevalent in men, and is associated with intestinal tumours and with so-called ‘unclassified’ gastric cancer.
- Microsatellite instability (MSI) – characterised by hypermutation. This subcategory is also called the methylator phenotype (CIMP). It is associated with intestinal cancers, less frequently with lymph node involvement, and is supposed to have better survival. It is more frequent in Asian countries and also in elderly patients. Available data indicates a prevalence of around 7.5%.
- Chromosomal instability (CIN). There are two subgroups in this subcategory. HER2+ cancers show overexpression of one of the EGFR receptors, and are associated with intestinal tumours, higher-grade tumours, and proximal tumour location. The other CIN variant is c-Met expressing, and is associated with higher tumour grade, proximal gastric cancers, and poor survival.
- Genomic stability (GS) – characterised by diffuse histology. This is probably the most challenging subgroup for treatment. The main molecular characteristic is presence of E-cadherin (CDH1) mutation. Integrins can also be affected, which are intercellular proteins and epithelial cell adhesion molecules (EpCAM).
After reviewing the field, Roecken recommended that histology remains important. He noted, however, that it is difficult to differentiate distal oesophageal adenocarcinoma from proximal gastric cancer based on classical histological criteria. He recommended that, in addition to classical histopathological work-up, every upper gastrointestinal cancer should be tested for MSI, EBV, HER2 overexpression and tumour mutational burden (TMB). However, he pointed out that there is, as yet, no standardisation of MSI definition in gastric cancer. Further validating work is needed before this becomes standard.
Question: In terms of treatment, there are phase II studies with checkpoint inhibitors in gastric cancer. Microsatellite instability and tumour mutational burden lack thresholds for which to recommend treatment with checkpoint inhibitors. In our institution we have started to measure MSI and TMB prospective for second- or third-line treatment. Would you recommend this also?
Answer: In our centre, we don’t have fluorescence in situ hybridisation (FISH) testing for EBV, and the method of testing was not mentioned in the talk at the conference. However, we now perform testing for mismatch repair proteins (MMR) and HER2 in every newly diagnosed gastric cancer, although we do not measure TMB. In the last three years I have had two elderly patients where I requested MMR testing, which came back deficient, and both responded remarkably well to checkpoint inhibition. So it seems feasible. However, we should be cautious and consider quality control when new tests are introduced.
Translating molecular subtyping into clinical practice
Pierre Laurent Puig (Paris, France), who has a particular interest in translational research, explored the theoretical and therapeutic aspects of the differences between oesophageal adenocarcinoma and squamous carcinoma and between oesophageal adenocarcinoma and gastric carcinoma.
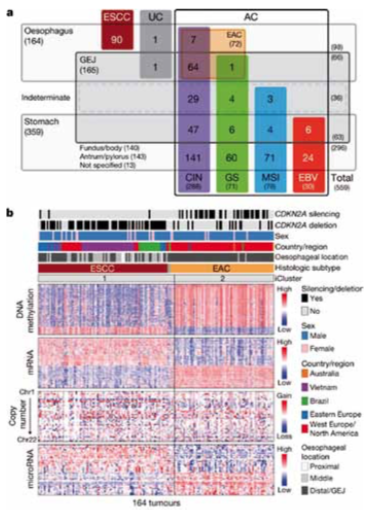
Similar to the 2014 Cancer Genome Atlas (TCGA) Network project for gastric cancer, a second project looked at 559 upper GI tumour samples (Nature 2017, 541:169–75). These comprised:
- 90 oesophageal squamous carcinomas
- 72 oesophageal adenocarcinomas
- 36 gastro-oesophageal junctional (GEJ) tumours of unknown origin
- 63 GEJ tumours
- 140 gastric carcinomas: fundus/body
- 143 gastric carcinomas: antrum/pylorus.
The study investigated gene alterations using different techniques, including methylation pattern, mRNA expression, microRNA and copy number alterations. Results showed high levels of DNA methylation in oesophageal adenocarcinomas (EAC; see figure, part b, right) which are rarely MSI high and generally chromosomal unstable. They showed low DNA methylation levels in squamous cancers (ESCC; part b, left)
Major genomic subdivisions of gastro-oesophageal cancer
The Cancer Genome Atlas research network categorised 559 oesophageal and gastric carcinoma tumours into sample sets (a). Integrated clustering of four molecular platforms (b) shows that oesophageal carcinomas fall into two molecular subtypes (iCluster 1 and iCluster 2) that are virtually identical to the histological classes oesophageal squamous cell carcinoma (ESCC) and oesophageal adenocarcinoma (EAC). Clinical data (top of b) and molecular data (bottom of b) from 164 tumours profiled with all four platforms are depicted
CIN ‒ chromosomal instability; EBV ‒ Epstein–Barr virus; GEJ ‒ gastro-oesophageal junction; GS ‒ genomically stable; MSI ‒ microsatellite instability; UC ‒ undifferentiated carcinoma
Source: The Cancer Genome Atlas research network (2017) Integrated genomic characterization of oesophageal carcinoma. Nature 541: 169–75, reproduced under a Creative Commons licence
The researchers analysed specific gene characteristics, revealing highlights that can be recognised from the clinic (see next figure). In the rectangle top left, results for squamous-type cancers (ESCC) showed amplification of EGFR, while results for adenocarcinomas (EAC) showed amplification of HER2 (ERBB2) as well as VEGFA, which is known to be amplified more frequently in adenocarcinoma than in squamous carcinoma.
Results for cell differentiation (middle right) showed a typical pattern for squamous cell carcinoma, with high levels of TP63, while this was rarely seen in adenocarcinoma. Cell cycle alterations (bottom left) were more frequent in squamous cell carcinomas, and there were also differences in mutational patterns.
Considering the differences between oesophageal adenocarcinoma and gastric carcinoma, the findings were relatively simple:
- Oesophageal squamous cell carcinoma had a stronger resemblance to head and neck squamous cell carcinoma than to oesophageal adenocarcinoma.
- Oesophageal adenocarcinomas more strongly resembled gastric cancers than oesophageal squamous cell carcinomas.
- Oesophageal adenocarcinomas and CIN gastric cancers jointly formed a group distinct from EBV, MSI or genomically stable (GS) tumours.
The conclusion from this presentation was that there is no distinction between gastric CIN tumours and distal adenocarcinomas. They have similar chromosomal aberrations. However, there is a progression of DNA methylation features from proximal to distal gastro-oesophageal adenocarcinoma-CIN tumours, which are most frequent in cluster 1 (eg CDKNA2) and with the lowest rate in cluster 4. Oesophageal adenocarcinomas have higher rates of mutation of SMARCA4 and deletion of tumour suppressor RUNX1, but lower APC mutation rates compared to gastric tumours.
Integrated molecular comparison of somatic alterations across oesophageal cancer types
Mutations and somatic copy number aberrations for selected genes and CDKN2A epigenetic silencing for oesophageal adenocarcinomas (EACs) and oesophageal squamous cell cancers (ESCCs) revealed many findings that can be recognised from the clinic. Genes are grouped by pathways, with lines and arrows showing pairwise molecular interactions. Alteration frequencies for each gene are listed inside rounded rectangles, with ESCC rates on the left and EAC on right, with red shading denoting gene activation, and blue denoting inactivation.
Source: The Cancer Genome Atlas research network (2017) Integrated genomic characterization of oesophageal carcinoma. Nature 541: 169–75, reproduced under a Creative Commons licence
Q: This confirms what we already knew in head and neck cancers. Yet there are, so far, no real implications for treatment. I am surprised that phase III studies do not differentiate between squamous cell carcinoma and adenocarcinoma in upper GI cancers, and treatment is not based on molecular subtype.
A: Even for something relatively simple such as EGFR amplification, this is not used in routine cases, although there are some data showing that EGFR inhibitors may have activity in squamous-type EGFR-amplified tumours. In addition, it is difficult to compare studies in patients from different ethnic groups and even within ethnic groups. The Cancer Genome Atlas data are not linked to clinical data, and there are no good outcome data that correlate genomic findings to clinical outcomes.
Classification of oesophago-gastric cancer: surgical facts and fiction
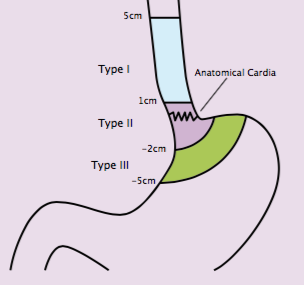
Paul Magnus Schneider (Zurich, Switzerland) considered the challenges of classifying oesophageal and gastric cancers from a surgical perspective. He suggested that the Siewert classification (see next figure) provides a surgical categorisation for oesophageal and gastric tumours based on anatomic and surgical indications. These were based on the extension of surgery by their location. Type I tumours, which are the most proximal, have better prognosis than type II, which in turn have better prognosis than type III. However, the prognostic calculations are potentially confounded by lack of stratification by treatment received. The value of this prognostic classification is therefore tempered. The most important element is the relationship to the extent of resection.
Siewart type I would generally be treated with oesophagectomy, while a distal oesophageal resection would be recommended for types II and III.
Schneider critically reviewed the Siewert classification at the same time as recognising its contribution to surgical treatment of upper GI cancers. He pointed out that the definition of the ‘zero point’ has changed over time, and there has been no independent validation of the classification system. The classification is defined pre-operatively, but the definitive classification is carried out intra- and postoperatively. He suggested that the system was developed around a standard of surgery that was valid at the time. Since then, however, surgery, including extensive surgery, has become safer, and recommendations may therefore be more aggressive. This is a particular issue for Siewert type II tumours, in the absence of any randomised trial of subtotal oesophagectomy.
A key issue is the accuracy and completeness of endoscopic reporting. Schneider warned that gastroenterology reports are often incomplete, missing important measures including the upper tumour border, the lower tumour border, the precise location of the Z-line, and fundus invasion status. Reports may also be missing information on the endosonographic upper and lower tumour borders. All of these measures are very relevant, confounding the comparability of data when reviewed retrospectively.
One of the surgical problems related to the Siewert classification is the extent of surgery. In Siewert I tumours, which are the most proximal, two-field adenectomy is recommended, resecting abdominal and mediastinal lymph nodes in addition to the primary tumour. For Siewert II, two-field adenectomy was initially recommended only in the lower media-stinum and abdomen. For Siewert III there is only one field, which is the abdomen. A study by Lerut et al. (Ann Surg 2004, 240:962–74) evaluated three-field adenectomy in Siewert type II tumours, which is larger than the two-field procedure. Results showed cervical lymph node involvement in 25%. This is of concern, suggesting that tumour may be left behind despite surgery.
Another issue is involvement of lymph nodes detected by PET scan. This applies particularly to para-aortic and celiac lymph nodes. If hypermetabolic lymph nodes are found on PET scan, is this an indication for surgery? An aggressive surgeon would consider it as such, but it may complicate surgery. A Japanese study comparing D2 (standard) with D3 (more extensive) adenectomy showed no benefit of more extensive adenectomy (NEJM 2008, 359:453–62). However, Schneider suggested this study may not be applicable to the Caucasian population, because tumours in this Japanese study were mainly proximal, whereas more distal tumours occur in the Caucasian population, and Siewert II and III tumours were underrepresented. Therefore, evaluation of more extensive lymph node involvement by PET remains mandatory.
The Siewert classification
The Siewert classification categorises tumours located near the oesophago-gastric junction according to anatomic and surgical indications
Which tumours need more than just surgery?
Everyone should have multimodal treatment, suggested Magnus Nilsson (Lund, Sweden) in a provocative answer to the question: which tumours need to be treated with more than just surgery?
Recognising the complexity of the issue, he reviewed the current perioperative treatment standard for gastric and oesophageal cancers based on the FLOT4 trial for gastric cancers (Lancet Oncol 2016, 17:1697–708; JCO 2017, 35 suppl. abstr. #4004), and the CROSS trial in oesophageal cancers (Lancet Oncol 2015, 16:1090–8).
A meta-analysis comparing neoadjuvant chemotherapy with neoadjuvant radiochemotherapy in oesophageal adenocarcinoma and squamous cell carcinoma showed that radiochemotherapy led to significantly better complete pathological response and R0 resection for both types of cancer (Eur J Cardiothorac Surg 2017, 51:421–31). However, significant three-year survival gains were seen only in squamous cell carcinomas. In adenocarcinomas, neoadjuvant radiochemotherapy did not show better survival rates than chemotherapy alone. Why is this? In the wake of this meta-analysis, the above-mentioned Swedish group of authors analysed the Swedish Cancer Registry for 900 patients treated between 2011 and 2015, comparing patients treated with surgery alone (n=500) with patients who received neoadjuvant chemotherapy (n=200) and those treated with neoadjuvant radiochemotherapy. Results showed increased morbidity and mortality with radiochemotherapy (Chin J Cancer Res 2017, 29:313–22). The treatment benefit from radiochemotherapy was mainly seen in patients with excellent performance status.
Nilsson concluded that use of a neoadjuvant treatment strategy should take the patient’s performance status into account. In patients with compromised performance, neoadjuvant chemotherapy or surgery alone may be preferred to neoadjuvant radiochemotherapy. This led to some debate at the St Gallen conference, with suggestions that there may be heterogeneity in the quality of surgery and variations in selection of patients for surgery. However, many patients are fragile, so it is important, particularly in those with squamous cell type cancers, to screen for cardiac or lung issues.
What imaging is needed to select patients for neoadjuvant treatment?
Angela Riddell (London, UK) reviewed the latest developments in imaging in GI cancers. She explained that the standard work-up for an upper GI cancer is an endoscopy and CT scan. Additional options include:
- Endoscopic ultrasound, which is important for T staging. The limitation is that stenotic tumours cannot be evaluated, as it is impossible to obtain a complete view.
- PET–CT, which Riddell recommended as a standard for defining tumour location and for detection of occult metastases. However, PET–CT scans show false-negative for diffuse type gastric cancer. A further concern is false-positives, which may lead to further, unnecessary, diagnostic work-up.
- Laparoscopy for peritoneal disease, where imaging notoriously underperforms.
Riddell then considered use of imaging in assessing response to chemotherapy. She reported that PET–CT has been validated for evaluating early response to treatment at day 14 after neoadjuvant chemotherapy (Gastroint Cancer Res 2008, 2:287–94). But there is concern about false-positives with PET. The ideal para-meters of response are still a matter of debate, with the MUNICON trial using maximum standardised uptake value (SUVmax) (Lancet Oncol 2007, 8:797–805). Other parameters such as metabolic tumour volume have not been evaluated extensively.
There were differences in opinion among the audience at the conference on the use of PET. Schneider and Riddell were adamant on the need for PET–CT, while others questioned its value. However, there are also questions of availability and personal practice. I consider PET–CT to be an important addition to providing information on the tumour.
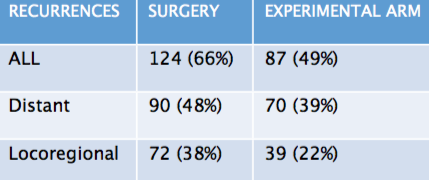
Pattern of recurrence in locally advanced oesophageal cancer
The results of the CROSS trial showed a reduction in both distant and locoregional recurrence with neoadjuvant
radiochemotherapy (experimental arm) compared to surgery aloneSource: V Oppedijk (2014) JCO 32:385–91
Patterns of recurrence to guide selection of multimodal treatment
Oesophageal cancer
Marcel Verheij (Amsterdam, the Netherlands) suggested that, in oesophageal cancer, the standard treatment for locally advanced disease was established by the CROSS trial (NEJM 2012, 366:2074–84). The trial included 368 patients with T1–T3/N1 cancers (75% adenocarcinoma, 25% squamous type) who were randomised to surgery alone or radiochemotherapy (41.4 Gy plus carboplatin AUC 2 with paclitaxel 50mg/m2) followed by surgery. Pathology results showed R0 (no cancer cells seen microscopically at the resection margin) was 69% in the surgery only arm and 92% in patients treated with radiochemotherapy prior to surgery (experimental arm). The complete pathological response rate was 29% in the experimental arm. The rate of lymph node involvement was 75% in the surgery only group and 31% in the experimental arm.
The overall rate of recurrence was 66% in the surgery group compared to 49% in the experimental arm (see table). Just under half of the patients treated with surgery alone (48%) had a distant recurrence, compared to 39% of those treated with radiochemotherapy followed by surgery. There was a significant reduction in the rate of locoregional recurrence, from 38% in the surgery arm to 22% in the experimental arm. Only 5% of locoregional recurrences occurred in the treatment field, showing that local treatment was extremely effective.
A study in 239 patients with T3/T4 tumours, which are inoperable, who were treated with radiochemotherapy (50.4 Gy, 28 fractions, plus 5FU) showed a higher recurrence rate of 50% for local failures and 48% for distal failures (Cancer 2012, 118:2632–40).
Gastric cancer
In gastric cancer, surgery (D2 disection) is the cornerstone of treatment. The rate of recurrence is 88% (two-thirds locoregional, one-third distant). With this high rate of recurrence there have been numerous efforts over the last few years to improve outcomes. Trials in the Caucasian population include the SWOG Intergroup Trial 0116 (also known as the Macdonald Protocol), which showed an overall survival benefit for postoperative radiotherapy (JCO 2012, 30:2327–33), and the MAGIC trial, which showed improved overall survival with perioperative chemotherapy (NEJM 2006, 355:11–20). In the Asian patient population, the ARTIST trial in Korea showed no difference in outcomes with postoperative radiotherapy compared to chemotherapy (JCO 2012, 30:268–73), while the CLASSIC trial gave an overall survival benefit with adjuvant chemotherapy (Lancet 2012, 379: 315–21). Outcomes were better in the Asian population, showing there are biological differences compared to Caucasian patients, and results cannot be compared.
The CRITICS trial (JCO 2016, doi:10.1200/JCO.2016.34.15_suppl.4000) has made a major contribution to the field, comparing a treatment regimen of chemotherapy–surgery–chemotherapy (the MAGIC approach) to chemotherapy–surgery–radiotherapy. It might have been assumed that use of all three treatment modalities could confer the greatest benefit. However, results showed no difference in the pattern of recurrence or overall survival between these two approaches. This indicated that there may not be an a priori role for radiotherapy after surgery for gastric cancer.
Looking ahead
Moving forward, Verveij noted that poor adherence is a major concern in gastric cancer trials. Even postoperatively, adherence to chemotherapy is often below 50% in clinical trials. He suggested that future trials should focus on improving adherence in the preoperative phase, in addition to efforts to improve sensitivity to treatment. Two ongoing trials are investigating these approaches:
TOPGEAR is comparing neoadjuvant chemotherapy followed by surgery and chemotherapy with chemotherapy–radiotherapy–surgery–chemotherapy.
CRITICS II is comparing three arms: chemotherapy–surgery, chemo-therapy–radiotherapy–surgery and radio—therapy–surgery.





Leave a Reply