Is there an early stage in the metastatic process at which the possibility of a cure remains open? Marc Beishon looks at the science, the opportunities opened up by new and more accurate imaging and treatment modalities, and the clinical evidence.
Can we cure any patients once their cancer has spread? It’s a question that oncologists have been asking for some time, and are still asking. The prognosis for most advanced solid tumours remains gloomy and, in the majority of cases, it is the metastases that kill.
Testicular cancer has, for many decades, been one of the rare exceptions to the rule, with effective chemotherapy regimens leading to survival rates of almost 75%, even among patients diagnosed with the most advanced disease (stage 3c). Increasingly there is promise now with new immunotherapies in melanoma and lung cancer, but so far only in relatively few patients.
Apart from systemic therapies – which are the mainstays of treatment for advanced cancers – surgery, radiotherapy, and other ablative techniques have also been used for a long time to treat and remove ‘mets’ in sites such as the liver, lung and brain, to relieve symptoms and/or extend life.
A minority of patients treated in this way have gone on to live a normal life span. The question is whether this proportion can be increased by bringing new biological knowledge, imaging and operative/ablative techniques to bear on what has become known as ‘oligometastatic cancer’ – a relatively recent term that means limited metastatic spread, usually to only one or two sites, which by definition is eligible for curative treatment.
‘Oligos’ is Greek for ‘few’, and the term oligometastasis typically refers to fewer than five mets, but there is no hard-and-fast definition, and the concept has been the source of a good deal of confusion and indeed scepticism that it represents any sort of definable clinical entity. As Socrates said, “The beginning of wisdom is the definition of terms.”
What are we talking about?
The term ‘oligometastasis’ was first used by Samuel Hellman and Ralph Weichselbaum in a paper published in the Journal of Clinical Oncology in 1995 (vol 13, p 8). In 2011, the same authors reviewed how thinking about the concept had developed over the intervening 16 years, in ‘Oligometastases revisited’ (Nat Rev Clin Oncol 2011, 8:378–82). Their proposal was that evolution of metastatic capacity has an intermediate status in which spread may be limited to specific organs, and mets might be present in small numbers – the clinical implication being that local treatments can be curative, as borne out by studies on spread to the liver, lung, and adrenal gland.
But the key question they posed was the prevalence of oligometastasis – if more patients with this ‘status’ could be identified, maybe through new biomarkers and molecular diagnostics, then the curative population could rise. Access to better ways of treating such local tumours would also be important – the authors mentioned in particular stereotactic body radiotherapy (SBRT), which can treat mets in multiple organs in a patient, including some mets not eligible for surgery.
Fast forward again to 2018, and Weichselbaum was honoured at the ASCO meeting in Chicago, where he gave the annual Karnofsky lecture (an article based on his talk was published in the Journal of Clinical Oncology, doi:10.1200/JCO.18.00847). Here he had a more detailed answer to how common the oligometastatic state is, noting though that this is still hard to pick out of current literature. Some people say one in ten cases, others one in three – but his group had picked one in five, based on the first clinical trial they did.
Speaking about the US, he said: “If you look at the four most common cancers, 90,000 patients a year either developed oligometastases or presented with them. If we include less-common tumours, like sarcomas and renal cancer, where the presentation is frequently metastatic, this is more than 100,000 patients a year. So this subset of cancers would make the potentially life-threatening cancers more common than any cancer except lung cancer.”
Put in those terms, oligometastasis has the potential to be much more important than “rare exceptions to the cancer metastatic paradigm”, as Hellman and Weichselbaum characterised conventional thinking in their 2011 Nature paper.
Put in those terms, oligometastases could be much more important than the ‘rare exceptions’ they were initially characterised to be
Like a lot of researchers, they are hoping to overturn that paradigm by understanding metastasis, and argue that it is a wide spectrum of disease in three respects: by the number of mets, by the organs they appear in and, importantly, by the pace at which they appear.
Weichselbaum argues that, contrary to many people’s conceptualisation of metastasis, the process is inefficient and often slow, as tumour cells have to detach and burrow into blood vessels and survive in the circulation and then move back out of vessels and colonise other sites, governed by genes and proteins.
One of the first studies they did found that the pace of recurrence of mets was a critical factor. In a set of patients with operable lung cancer, with between one and five mets at follow-up, there was a huge difference in survival for those who presented at a rate of fewer than 0.6 mets a year and those who developed more than 3.6 mets a year – it was mostly a difference between life and death.
Precision in targeting oligometastases
It is the combination of imaging, local ablative techniques (especially stereotactic body radiotherapy), and development of biomarkers that is fuelling the work on oligometastasis.
Imaging
As a recent review by the imaging group of the EORTC (European Organisation for Research and Treatment of Cancer) reports, correct identification of oligometastatic disease is not trivial, and whole-body in vivo imaging is the only realistic current option for detection (Eur J Cancer 2018, 91:153–163). Advanced imaging modalities – especially using PET-CT – are starting to supersede standard ones (CT, MRI, bone scintigraphy). For example, a PSMA (prostate specific membrane antigen) tracer with PET-CT targets a protein expressed in prostate cancer (also expressed in other cancers such as kidney and liver). “It allows us to see affected lymph nodes and lesions we just couldn’t see a few years ago,” says radiation oncologist Alan Dal Pra. Piet Ost and colleagues, in their paper on the STOMP trial (see “Surveillance vs metastasis-directed therapy in oligometastatic prostate cancer” box), also note that the PSMA tracer, specifically 68Ga-PSMA, holds great promise – they used choline PET-CT imaging in their study instead, which was the tracer available in Belgium at that time, but is outperformed by the PSMA tracer at low PSA levels. The PSMA–PET modality is now in wide use in many countries (see also review, Eur Urol 2018, 74:179–90, which found that using 68Ga-PSMA PET altered management of about half of patients with metastatic prostate cancer).Stereotactic body radiotherapy (SBRT)
EORTC, together with ESTRO (the European Society for Radiotherapy and Oncology), is conducting a ‘basket’ observational registry trial called OligoCare to collect outcomes on oligometastatic patients treated with SBRT (see slides at OligoCare). It is noted that SBRT is a standard of care, “despite a lack of hard evidence and despite huge uncertainties and variability in practice”, and that traditional clinical trials won’t provide all the answers.There are several machines that can deliver SBRT, including conventional linear accelerators, tomotherapy, cyberknife and MRI-guided radiotherapy, as well as gamma knife, which is used only for the treatment of cranial lesions.
Along with surgery there are other local ablative approaches – in the liver, in both operable and inoperable settings, there is radiofrequency, selective internal radiation therapy (SIRT), portal vein emobolisation, and embolisation using chemotherapy. But SBRT has found favour among many radiation oncologists for a range of tumour sites (see for example Cancer Treat Rev 2017, 52:22–32).
Biomarkers
Work on finding biomarkers that will identify which patients with oligometastases are likely to benefit from local intervention is in its early stages, and is allied to the large body of research on widespread metastatic disease, including on circulating tumour cells and DNA. In addition to the recent work on molecular subtyping of liver mets noted in the main text, there is research on microRNAs (miRNAs) as genetic probes for distinguishing between oligometastatic and polymetastatic (>5) lung cancer mets, as current imaging methods are said to be insufficient (see Medicine (Baltimore) 2018, 97:e10958). There has also been a study on gene signatures of lung mets from kidney cancer (Int J Cancer 2009, 125:474–482).
Who should be eligible for intervention?
Researchers at a number of centres have since been looking at the clinical and biological factors that could determine whether a patient could have a good outlook from an oligometastatic intervention, and there has been a small number of randomised controlled trials (RCTs). This year, Weichselbaum and colleagues published a paper that details molecular subtypes in colorectal cancer that can categorise patients into low-, intermediate- and high-risk groups for liver metastases (Nature Comm 2018, 9:1793).
Peter Naredi, a surgical oncologist at Sahlgrenska University Hospital, in Gothenburg, Sweden, and a specialist in liver and gastrointestinal cancers, argues that immunotherapies, which are resulting in long-lasting durable responses in a minority of patients, are changing the perception of many in the oncology community about the curability of some metastatic disease. “But we have been talking about this with surgery for years,” says Naredi. “We can get long survival if we chose the right cases, particularly for liver metastases that arise from colorectal cancer, and also from the much rarer neuroendocrine tumours.”
At present, he says, one in two patients deemed eligible for liver surgery has an excellent chance of five-year survival – but only if there are modern operative techniques that, for example, minimise bleeding, and excellent perioperative care, that can result in good quality of life. There is an existing knowledge base that, when coupled with multidisciplinary care, can provide this one-in-two chance, but Naredi says that, even in Sweden, patients living near a university hospital are more likely to be referred for such selection than those in out-lying hospitals. A study from the UK, he notes, looked at National Health Service hospitals and found a wide variation between rates of surgery for liver metastases. Notably, it also found that, where such surgery was done on colorectal cancer patients, outcomes were equivalent to those with stage 3 colorectal cancer (lymph node involvement but not metastatic) (see Br J Surg 2010, 97:1110–18).
There is a lot at stake. Naredi says about two in every five colorectal cancer patients develop liver metastases, and about one in five, or even one in four of them, should be eligible for surgery. That could be up to 10% of the colorectal cancer population. “If I say to patients they have a 50% or possibly better chance of five-year survival, they want that chance,” says Naredi.
Precision planning for local treatment
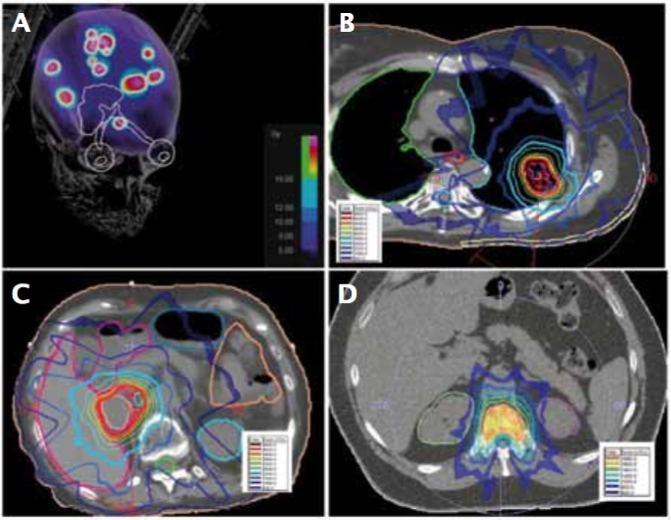
Examples of stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT) treatment plans for melanoma metastasis: A) SRS for multiple brain metastases; B) SBRT for lung metastasis; C) SBRT for adrenal metastasis; and D) SBRT for spine metastasis
Source: W Shi Radiation Therapy for Melanoma. In: WH Ward and JM Farma eds. (2017) Cutaneous Melanoma: Etiology and Therapy [Internet]. Brisbane (AU): Codon Publications, Chapter 8. Available from: https://www.ncbi.nlm.nih.gov/books/NBK481863/ doi: 10.15586/codon.cutaneousmelanoma.2017.ch8. Published under a Creative Commons licence
Guidelines, evidence and clinical trials
In mainstream clinical practice, Naredi considers that only colorectal/neuroendocrine liver mets have a sufficiently high level of evidence to justify curative surgical intervention. He mentions ongoing research using registry data on patient selection according to factors such as age and comorbidities, on the nature of the primary and metastatic tumours, and on adding drug treatment, such as using chemotherapy, to shrink tumours before surgery or to prolong life after surgery.
Naredi sees two main problems in current practice – a failure to refer patients to expert centres for assessment, and overtreatment by surgeons who perform non-evidence-based resections on metastases in a range of cancer types, such as pancreatic cancer. Guidelines are urgently needed to rectify both problems, he says.
“There are a number of surgeons in Europe who are carrying out what is essentially futile surgery because they don’t want to tell the patient the truth, leading them to believe they can cure their cancer. We do have case reports on, for example, liver resections for pancreatic cancer, but we have seen no long-term survival. In my view it is wrong to do such surgery outside of clinical trials in cancers such as pancreatic and oesophageal, and national guidelines need to stress this.”
Naredi adds that there is emerging evidence for a middle category of cancers, such as breast, prostate, melanoma, sarcoma, and ovarian cancer, to show that surgery (or stereotactic body radiotherapy and other ablative techniques such as radiofrequency) can be an option, where there is evidence of an oligometastatic state.
The field is also moving on to examining multimodal therapies, including chemotherapy, radiation, and new biological therapies, as well as surgery. But the less flashy, pain-staking work involved in defining which groups of metastatic patients might benefit from different types of local ablative therapy already in mainstream use is quietly proceeding, says Naredi, even though it is not the type of work that is likely to hit headlines or make careers.
What all this also demands – from what should be a standard referral for liver mets, to the intermediate and cutting edge work – is working in multidisciplinary teams, as Naredi reiterates. In the metastatic setting, for a long time too many patients have been referred to isolated medical oncologists for the possibility of systemic therapy, but the wide choice of options for both curative and non-curative approaches, as detailed in numerous recent papers, demands an MDT that is on top of the current research. It should no longer be acceptable for patients diagnosed with metastatic disease to be referred automatically to management by medical oncology alone.
Breast cancer
The MDT point was also highlighted in 2017 in an abstract in The Breast, written by a team at the Netherlands Cancer Institute, in Amsterdam, concerning questions raised about the best treatment for one of their patients – a 38-year-old woman who had triple-negative breast cancer and presented with two liver lesions six years after primary treatment (vol 36, p S60).
The wide choice of options for both curative and non- curative approaches demands an MDT that is on top of current research
These included whether there are biomarkers to help select patients with oligometastatic breast cancer who might benefit from a multidisciplinary approach; the preferred method for local treatment (surgery, stereotactic radiotherapy, radiofrequency ablation, combinations); the extent of radiological remission following chemotherapy that should be required before proceeding to local treatment; and how the patient should be followed up. (The patient was treated with chemotherapy, followed by surgery, and at the time of publication she had been free of clinical or radiological signs of cancer for four years.)
The range of questions make clear that expert input from most of the core members of the multidisciplinary team needs to be brought to bear – medical oncologists, surgeons, radiologists (including interventional specialists), radiation oncologists, and pathologists.
There’s a sense of frustration though at the slow pace of progress. The Amsterdam team notes that oligometastatic breast cancer was discussed at the 2008 meeting of the European School of Oncology Metastatic Breast Cancer Task Force (JNCI 2010, 102:456–63). That meeting called for prospective RCTs to generate robust evidence, and yet ten years on, the most recent guidelines from the Lisbon Advanced Breast Cancer (ABC) conference continue to say that, while curative treatment should be considered for selected oligometastatic patients, a prospective clinical trial is still needed.
The Dutch team mentions that they are conducting their own prospective study, in which patients with oligometastatic breast cancer are treated with ‘neoadjuvant’ chemotherapy and maximal local therapy for all detected metastases and locoregional disease, and they advocate for pooling efforts to create a prospective registry for patients with oligometastatic breast cancer across Europe. The most urgent questions flagged up by the registry could then be investigated in an international trial led by the EORTC, they suggest.
Prostate cancer
Prostate cancer is another area where interest is growing in identifying an intermediate or oligometastatic state between patients with localised disease and more widespread metastatic disease. “This is also because we have new imaging modalities that can detect lesions more accurately,” says Alan Dal Pra, a radiation oncologist at the Sylvester Comprehensive Cancer Center, in Miami, Florida. “We also have the ability to treat the lesions with low toxicity, using stereotactic radiotherapy – just one to three fractions are needed,” (see also box on imaging and SBRT).
Surveillance vs metastasis-directed therapy in oligometastatic prostate cancer
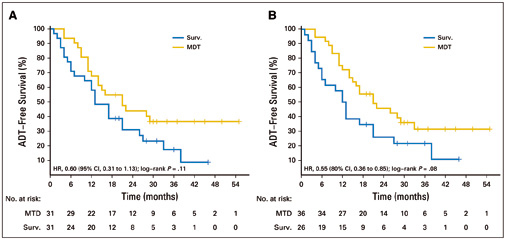
In the phase II multicentre STOMP trial, 62 men with asymptomatic prostate cancer with biochemical recurrence after primary treatment with curative intent, and three or fewer extracranial metastatic lesions, were randomised to surveillance or metastasis-directed therapy (MDT) of all detected lesions. The treatment was either by surgery or stereotactic body radiotherapy. The primary endpoint was androgen deprivation therapy (ADT)–free survival. ADT was started at symptomatic progression, progression to more than three metastases, or local progression of known metastases. At a median follow-up time of three years, the median ADT-free survival was 13 months (80% CI=12‒17 months) for the surveillance group and 21 months (80% CI=14‒29 months) for the MDT group (HR 0.60, 80% CI=0.40‒0.90, log-rank P=0.11). Quality of life was similar between arms at baseline and remained comparable at 3-month and 1-year follow-up. Six patients developed grade 1 toxicity in the MDT arm. No grade 2‒5 toxicity was observed.
Figure A shows analysis by intention to treat; Figure B shows per protocol analysis. The surveillance arm (Surv) is in blue, the metastasis-directed therapy arm (MDT) is in gold. Source: Piet Ost, Dries Reynders, Karel Decaestecker, et al (2018) JCO 36:446-453. Reprinted by permission from the American Society of Clincal Oncology. © 2017 ASCO
Dal Pra points to a phase II RCT led by Piet Ost, at Ghent in Belgium, called STOMP, which reported this year (JCO 2018, 36:446–53; see next box). It assigned 62 men with three or fewer metastatic lesions to either treatment of the lesions or surveillance. The men were then monitored until they required androgen deprivation therapy (ADT), the standard therapy given when the cancer progresses. The median ADT-free survival time in the intervention group was more than 50% longer than that of the surveillance group, at 21 months compared with 13 months.
One remarkable finding, which Dal Pra also notes, was that the 35% of patients in the surveillance arm experienced spontaneous PSA declines (the marker used to gauge progression) without receiving any therapy, although for most this did not last. But it supports the concept of oligometastasis – that certain tumours have not fully developed their metastatic potential and show a slow natural history, say Ost and colleagues.
The prostate study also raises the question of lead time bias – whether intervening earlier does confer benefit or if survival would be the same. The results from the STOMP trial suggest that there is real benefit. The issue was discussed by clinicians in Greece in a paper, ‘Oligometastatic prostate cancer: is it real?’ (J Cancer Prev Curr Res 2017, 8:00295). Apart from aptly quoting Socrates on getting definitions right, they report that treating such mets does appear to decrease the need for subsequent palliative care – so it is a real state – and also that toxicity rates are low, which is another important factor in deciding whether to give local treatments. Other commentators, including Weichselbaum, have also noted the ‘immortal lead time bias’, in that it is selected patients who may do well in single-arm studies, and so well-designed RCTs are crucial (see Nat Rev Clin Oncol 2014, 11:549–557; and J Targeted Therapies Cancer, 2018, 27 April).
While the STOMP study may not sound as exciting as the immunotherapy stories, those in the oligometastatic field recognise it as an important step in realising the potential of such intervention, certainly in prostate cancer, and Ost and colleagues feel it justifies moving to a phase III trial.
A related and well-known trial, STAMPEDE, which is adding other therapies to hormone therapy, has reported survival benefits for localised low metastatic burden when radiotherapy to the prostate is added, and the authors ask if there would be further benefit from additional radiotherapy to the oligometastases themselves.
Dal Pra adds that the Movember Foundation is funding an initiative under its sixth global action plan with at least 16 groups, including his own, to pool knowledge and samples from existing and planned trial work on treating intermediate spread prostate cancer. One of the problems with current research efforts, he notes, is that trial designs have a high degree of variability concerning the definition of oligometastasis, treatment technology, outcome measurement, and imaging methods. The aim of the Movember initiative is to build on retrospective and prospective clinical trials and invest in a complementary translational research project to answer critical clinical questions regarding tumour heterogeneity and treatment response.
Melanoma
In melanoma, Don Morton at the John Wayne Cancer Institute in Santa Monica, California, has for many years carried out surgery on metastases and has shown good long-term survival, despite opposition from medical oncologists, notes Naredi. In 2015, the group at John Wayne reported on a 45-year history of cure for melanoma metastases to the abdomen, where they argued that, even in the era of immunotherapy for advanced melanoma, surgery still offered a better opportunity for long-term survival than systemic therapy.
Lung cancer
In lung cancer, Weichselbaum reported on several papers, including a review in The Lancet on the use of stereotactic body radiotherapy (SBRT) to treat lung mets in a number of studies, showing that 20–30% of patients with limited disease were cured.
A later meta-analysis of lung cancer patients found an “astounding” near-50% five-year survival for a certain group. And Weichselbaum also noted several small RCTs, including one on lung cancer, which were stopped because the results were so much better in the ablative group that the investigators thought it was unethical to continue.
That study was led by Puneeth Iyengar at the University of Texas Southwestern Medical Center, who has also co-authored a number of other papers including one in 2018 that reviewed oligometastatic and oligo-progressive lung cancer (J Thorac Dis 2018, 10:S2537–44).
It seems from this review, and from a rapidly growing list of papers on lung cancer, that the usual perception of all metastatic lung cancer as being incurable is being robustly challenged, but big knowledge gaps remain to be filled, and again this will only be advanced by research-oriented MDTs.
Sarcoma
Another cancer type where it would be hard to imagine any treatment proceeding without MDTs is sarcoma, which has many subtypes and is highly complex. A number of groups have addressed oligometastatic disease in sarcoma, especially in the lung, where about half of soft tissue sarcomas metastasise.
Head and neck cancer
A recent review of selected patients with recurrent/metastatic head and neck squamous cell cancer undergoing surgery or SBRT shows five-year survival rates of more than 20%, compared with median survival of about 10 months after first-line systemic treatment.
The authors say what is needed are revised imaging follow-up strategies to detect mets earlier; identification of predictive noninvasive biomarkers to guide treatment; assessment and corrections of biases in current studies; and, of course, RCTs (Future Oncol 2018, 14:877–889).
A new era?
A point stressed by Weichselbaum is that there is merit in thinking further than just a few lesions. He and others are now pushing the boundaries to address more advanced conditions, which include the presence of more than five metastases, the presence of ‘oligoprogression’ – where some lesions are progressing and not stable – and even more widespread disease.
In his round-up of the brief number of RCTs, Weichselbaum mentioned one led by Theo Ruers, a surgeon at the Netherlands Cancer Institute, on the long-term effects of applying radiofrequency ablation plus chemotherapy as an aggressive method of treating up to nine inoperable colorectal liver mets, versus systemic therapy alone, finding a significant survival advantage in the ablation group (JNCI 2017, 109:djx015). This is the first such phase II trial.
There is also impressive news from a recent multicentre randomised phase II SBRT study, known as SABR-COMET, which reported recently at the annual meeting of the American Society for Radiation Oncology (ASTRO). It looked at the impact of adding SBRT to standard palliative care in patients with up to five mets from mainly breast, lung, colorectal, and prostate primaries. It found a greater than expected median survival in the SBRT arm of 41 months vs 26 months, and a doubling of progression free survival to 12 months. It is the first trial to demonstrate a survival benefit, says Dal Pra, and so helps address the lead-time bias issue. Nearly half (46%) of the patients treated with SBRT were still alive after five years, compared with 24% in the control group. A follow-up study will enrol patients with up to ten mets.
“Oligo is just a subset, a lower bound of metastasis,” Weichselbaum says. “Now we want to see if maybe, combined with other therapies, we could treat 10 or 20. “So the conclusion is that some patients have oligometastatic disease and can be cured with ablative therapy. These patients can be identified through clinical features and molecular parameters. Some patients with oligoprogressive disease might be cured, and what about more widespread disease?”
“Now we want to see if maybe, combined with other therapies, we could treat 10 or 20”
That’s where immunotherapy, including T-cell therapies, possibly combined with radiation (which can stimulate the immune system), chemotherapy, and targeted therapies, may be coming into play, with the application of a lot of advanced thinking on molecular biology.
Perhaps the most important message from the work is that a systematic era of ‘metastatic-directed therapy’ (another MDT abbreviation) is emerging, as researchers put together risk classifications and appropriate multi-modal treatments for patients who would have received only lines of drug therapy to control their advanced cancer. Landmark studies, such as that by Weichselbaum and colleagues on the molecular subtyping of liver mets, could also help convince the sceptics that we are dealing with real and unique entities in cancer.
Clinical trials
This selection of new and ongoing trials is from a total of about 80 that mention ‘oligometastatic’ or similar on ClinicalTrials.gov. There are several thousand trials concerning metastasis, some of which will also have relevance to oligometastasis, such as in ablative techniques.
SARON – phase III study on efficacy and safety of stereotactic body radiotherapy (SBRT) and conventional radiotherapy alongside standard chemotherapy in patients with oligometastatic lung cancer. Guys & St Thomas, and others, UK.
CORE: Phase II/III RCT in patients with breast, prostate or lung cancer comparing standard of care with or without SBRT for extracranial metastases. The Royal Mardsen, London.
Multicentre adaptive phase II/III randomised trial of SBRT in oligometastatic castration-resistant prostate cancer patients. Jewish General Hospital, Montréal.
Standard of care with or without SBRT and/or surgery in limited metastatic breast cancer. Phase IIR/III trial at 136 international locations. NRG Oncology, Philadelphia.
FORCE – focal radiation for oligometastatic castration-resistant prostate cancer. Phase II RCT. University of Michigan Cancer Center, Ann Arbor.
PEACE V: Phase II RCT for salvage treatment of oligorecurrent nodal prostate cancer metastases. University Hospital, Ghent, and others in Belgium and Europe.
Phase II RCT on how well systemic therapy with or without local consolidative therapy works in treating participants with a solid tumour that has spread to one site. Banner MD Anderson Cancer Center, Gilbert, Arizona.
Single-arm prospective phase II study of SBRT for oligometastases from colorectal cancer. Cancer Hospital, Chinese Academy of Medical Sciences, Beijing.
ORIOLE: SBRT for prostate oligometastases randomised against observation. Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins, Baltimore.
Local therapies for oligometastatic lung cancer harbouring sensitising EGFR mutations. Memorial Sloan Kettering, New York.
Cohort study to explore prognoses of lung cancer patients with oligometastases. Multiple centres in China.
High-dose chemotherapy in oligometastatic homologous recombination deficient breast cancer. NKI-AVL, Amsterdam.
SBRT for inoperable lung and liver oligometastases from breast cancer. Istituto Clinico Humanitas, Milan.
Apatinib combined with SBRT in breast cancer oligometastasis. West China Hospital, Chengdu.
Chemoradiation +/- surgery versus systemic therapy for oesophageal or gastric cancer with oligometastases. MD Anderson, Houston.