Health systems and insurances take decisions on reimbursing targeted medicines, doctors focus increasingly on tailoring treatments to individual patients and the molecular biology of their disease. But the need for funded quality-controlled services to do the testing required for the tailoring has been largely overlooked, as Janet Fricker reports.
Biomarker testing is key to personalising treatments, helping doctors protect their patients from therapies that will do them more harm than good, and facilitating sustainable access to the right therapeutic strategies for those who will benefit.
With governments across Europe scrambling to find ways to maximise the therapeutic value they get for their money, ensuring access to testing in cases where it can better inform treatment decisions would seem an obvious step to take.
Yet as a steady stream of costly new therapies continue to make their way onto the market, access to tests that could help identify the minority of patients who could benefit remains extremely patchy across Europe.
France and England, which both have highly centralised healthcare systems and invest heavily in research, are set to introduce platforms, funded by their respective health services, that offer whole genome sequencing to cancer patients. However, most other European countries, including Germany, Spain, Italy, and Poland, have no such centralised initiatives even for testing specific biomarkers or panels of biomarkers. Instead, cancer patients face a healthcare lottery, where access to biomarker testing is largely determined by how engaged their individual clinicians are with the concept of genomic medicine, and whether they have championed the cause and sorted out funding.
“We need to help politicians recognise that testing is as important as the drug”
Fabrice André chairs the European Society for Medical Oncology (ESMO) Translational Research and Personalised Medicine Working Group, and is a professor in the Department of Medical Oncology, at the Institut Gustave Roussy, Paris. “When governments take the initiative in setting up genomic testing services, they provide new models of access with accompanying funds. But beyond government schemes there is currently no real access to biomarker testing across Europe,” he says, adding that clinicians are forced to rely on negotiating money from hospital drug budgets, or obtaining funding from charities supporting biomarker testing, or even asking patients to pay for their own tests.
Heinz Zwierzina, chair of the Cancer Drug Development Forum, from Innsbruck Medical University, Austria, agrees that governments need to step up and take responsibility in this area. “What governments are failing to realise is that to give patients across Europe equal access to precision medicine drugs they need to provide equal access to companion diagnostics. Without this in place we’re in danger of operating an immensely unjust health system,” he says.
Francesco De Lorenzo, President of the European Cancer Patient Coalition (ECPC), concurs that health departments have largely overlooked the challenge of providing access to companion diagnostics “We need to help politicians recognise that testing is as important as the drug. If these issues are not sorted out they will cause enormous suffering to patients, who will be exposed to the unnecessary toxicity of drugs they’ve no chance of responding to,” he says.
In the long term, he adds, lack of testing will result in unsustainable healthcare systems.
Setting up a national testing service
For a national biomarker testing service, the first decision that needs to be taken is the type of testing provided. Whether it makes sense to focus on more limited panels of biomarkers or go for whole genome sequencing, which picks up every mutation in the DNA of the tumour sample, represents one of the most hotly debated issues.
Andrew Hughes heads up the experimental cancer medicine team at the Christie hospital, a leading cancer centre in Manchester, UK. He argues strongly in favour of tests that look for a limited number of biomarkers, such as the Manchester Genomic Panel, developed by the Manchester Centre for Genomic Medicine, which tests for 24 biomarkers linked to treatments.
“It’s hardly surprising that if you look for more needles in the haystack you’ll find them. But the question is whether you’ll understand the significance of all the information you unearth,” he argues. “Why spend time and money to find genomic alterations for which you don’t have treatment options?”
Nirupa Murugaesu, from the 100,000 Genome Project in the UK, disagrees. “Later this year, a subset of cancers will have whole genome sequencing commissioned by the NHS [National Health Service]. Currently the majority of testing is via cancer panels, but it’s anticipated we’ll soon reach a ‘tipping point’, where the cost of whole genome sequencing, and the increasing evidence for pan-genomic markers such as tumour mutational burden and signatures, make it a more pragmatic choice.”
The big advantage of whole genome sequencing, she adds, is that it ‘future-proofs’ patients when new targets are detected, and also provides invaluable information about mutational burden, which is now believed to predict for good responses to immunotherapy.
Recent studies in non-small-cell lung cancer by Matthew Hellman and colleagues, from Memorial Sloan Kettering Cancer Center, New York, indicate that tumour mutations burden, found using whole-exome sequencing, predicts response to combination immunotherapy of PD-1 plus CTLA blockade (Cancer Cell 2018, 33:853–61; NEJM 2018, 378:2093–104).
 Waiting for better evidence
Waiting for better evidence
Perhaps the greatest barrier to investing heavily in national genomic testing services and integrating them into the health system, in the way France is now doing, stems from scepticism that the overall approach to treating cancer or other diseases based on their genomic characteristics will ultimately prove a fruitful way forward for a sizeable proportion of patients.
“While targeted therapies have been shown to work in different cancer indications, undoubtedly a major stumbling block for countries like Germany is that no studies have shown that the personalised medicine paradigm, where patients are allocated drugs according to genomic testing, improves survival,” says Christof von Kalle, from the National Centre for Tumour Diseases, Heidelberg.
He refers to the SHIVA01 trial, the first prospective randomised trial to evaluate the strategy of precision medicine. That trial, in which patients were randomised to treatment selected on the basis of tumour profiling (the experimental arm) or to physician’s choice, failed to show any difference between the two arms for the primary endpoint of progression-free survival (Lancet Oncology 2015, 16:1324–34).
“We want to help clinicians understand what’s important and what’s not”
Christophe Le Tourneau, the principal author of SHIVA01, from Institut Curie, Paris, says, “SHIVA01 shows that it’s not that simple treating patients in a histology agnostic way, and that precision drugs may not work in different molecular landscapes.” Vassilis Golfinopoulos, Headquarters Director at the EORTC, warns, however, about the longevity of these results. “While such trials need to be undertaken, results are only valid for a short period, because testing technology is continually evolving. Additionally, the number of targeted drugs is also increasing, with the possibility that a critical mass will soon be reached where they can make a difference on a global scale,” he says.
Once mutations have been identified from testing, particularly if more than one is identified, questions remain around how clinicians will unravel which to target first. “Currently it often boils down to a pragmatic approach around patient preferences, taking into account things like side-effects,” said Hughes, from the Christie cancer centre.
In an effort to help answer those questions, ESMO recently published a consensus-based ‘Scale for Clinical Actionability of molecular Targets’ (Ann Oncol 2018, doi:10.1093/annonc/mdy263). “We want to help oncologists to navigate these new clinical pathways. When they identify a number of different mutations in the same sample, we want to help them to understand what’s important and what’s not, and which has the highest evidence to target first,” says Fabrice André.
Whole genome sequencing or a panel test?
Knowledge of tumour genomic changes, such as those obtained through large-scale international tumour sequencing projects, has enabled the development of targeted drugs to switch off mutated oncogenes. The paradigm of targeted therapy (first exemplified with the US FDA’s approval of Herceptin in 1998), has been repeated with many other targeted agents since. More recent examples include: ALK inhibitors, such as crizotinib, alectinib or ceritinib, to target non-small-cell lung cancers with an ALK rearrangement; vemurafenib or dabrafenib to target melanomas with the BRAF V600E mutation; and olaparib and rucaparib, which target a protein involved in DNA repair that is important for cancers associated with alterations in the BRCA1/2 genes.
Early biomarker testing analysed single mutations, looking to see whether patients had the specific gene that could be targeted by single specific drugs. But as more biomarkers have become clinically actionable, using multiple single tests became unfeasible, leading to the development of panels of gene assays that are becoming ever more sophisticated. Current examples include the Manchester Genomic Panel, profiling 24 genes linked to treatments, and the FoundationOne test, which profiles 315 genes known to be associated with malignancies.
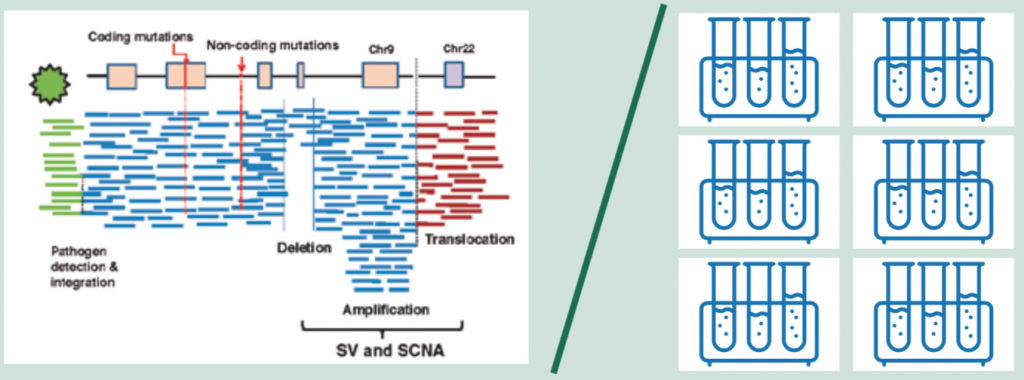
However, the single all-encompassing test of whole genome sequencing (WGS), which now costs around $1000 and can be turned round in a day, is becoming ever more feasible. WGS provides a base-by-base view of genomic alterations, looking at all 3.2 billion letters of the code. In addition to protein-coding mutations it can also detect non-coding mutations, structural variants (SVs) including SCNAs (somatic copy number alterations) and translocations, as well as pathogens (see figure above, left).
For cancer, a ‘paired’ approach is taken where the normal genome sequenced from the blood is subtracted from the tumour genome, allowing identification of acquired cancer mutations.
The information generated by WGS requires around 200GB storage space for one genome – around the size of an average laptop.
Source: H Nakagawa et al. (2015) Cancer whole-genome sequencing: present and future. Oncogene 34:5943‒50. Reprinted by permission from Springer Nature © 2015
A regulatory black hole
A major issue for companion diagnostics is that they currently fall under the European testing radar, being classified as ‘declared-tests’ according to the In Vitro Diagnostic Directive 98/79/EC (IVDD). While the European Medicines Agency (EMA) reviews the efficacy and quality of medicines, and the Conformité Européene (CE) considers medical devices (through over 900 different Notified Bodies located in different countries), there is no central agency in Europe with responsibility for reviewing the actual diagnostic tests.
This is set to change, however, with the In Vitro Diagnostic Regulation, which comes into effect in May 2022. The Regulation will require companion diagnostic tests to undergo Notified Body Review and EMA consultation. “This new process could result in a more harmonised review of companion diagnostic tests, and some level of connection with targeted therapy reviewed by the EMA,” says ECPC’s Lydia Makaroff.
The current knock-on effect of this lack of official testing means there is no evidence for health technology assessment bodies, such as NICE in the UK, to consider cost-effectiveness and make recommendations to health services regarding funding. The outcome is that, all too often, precision medicine drugs are licensed in Europe without the availability of the genomic tests that are vital to identify the patients most likely to benefit.
Where enlightened hospitals do offer genomic testing, they often appropriate the money from drug budgets (arguing the economic benefits of avoiding inappropriate therapy), or use charitable funding. In countries such as Spain and Italy, the pharmaceutical companies have taken on board the cost of testing, but this raises questions about whether bodies that have a vested interest in whether or not their drug is prescribed should be the ones to fund the testing.
“What’s really concerning about the lack of testing is that anyone in Europe can set up a testing service,” says Rafal Swierzewski, a Polish cancer patient advocate who represents ECPC on the EMA Committee for Medicinal Products for Human Use (CHMP). There is no requirement, he says, for companion diagnostic tests to be standardised, to ensure the results from any given specimen won’t vary according to which diagnostic facility does the testing.
Such a casual attitude towards companion diagnostics is symptomatic of the low priority afforded to medical testing in Europe. That is how David Brunel, from the US diagnostics company Biodesix sees it anyway. “In many European countries there is minimal recognition of the cost required to develop companion diagnostics alongside therapeutics, particularly if that effort is led by a diagnostic company with a novel approach and without pharmaceutical support.
“It therefore becomes difficult to obtain reimbursement for tests that justifies this investment and recognises the value they add to the broader health economic equation,” he argues. With such low rates of potential returns, he adds, there is a risk that US diagnostic companies will focus less on Europe, but instead target emerging markets, such as China, which may be more willing to pay a fair price.
The way forward for Europe, suggests Zwierzina, from the Cancer Drug Development Forum, would be for the EMA to take on board companion diagnostic testing.
This would be in line with the practice in the US, where the national regulatory authority, the FDA, has been responsible for regulating medical devices since 1975, and has developed guidance laying down the framework for co-approval of drugs and their companion diagnostics. The legislation due to come into effect in 2022 should be a step in that direction.
Setting up a national sequencing service
One of the biggest hurdles for establishing genetic testing services is developing the core infrastructures to underpin the service. Sophisticated systems need to be put in place, including: high-throughput sequencing facilities; ‘genome friendly’ pathways for tumour sampling (DNA can degrade using traditional formalin fixation); biobanks to store the tissue; capacity to manage the resulting massive digital data; and IT systems to return the results to clinicians.
Not least is the need to train a workforce of skilled professionals to interpret the science, and the establishment of multidisciplinary tumour boards to make sense of the data. “To seize the opportunities of personalised medicine requires in-depth expertise across clinical, genomic, health informatics, bioinformatics and social engagement and implementation fields. Many of the skills and experience are rare in individual countries and difficult to harness,” says Denis Horgan, Director of the European Alliance for Personalised Medicine.
The UK and France are leading efforts to set up national genetic sequencing services.France Génomique
The French Plan for Genomic Medicine 2025 is set to introduce high-throughput technology to allow substantial numbers of patients to receive personalised, diagnostic, prognostic and therapeutic care through sequencing of their genomes.
France Génomique aims to establish 12 sequencing platforms across the country, covering all diseases. The plan is to start by whole genome sequencing (WGS) of patients with rare diseases, forms of diabetes, and cancer. It is anticipated that France will be capable of sequencing 235,000 genomes per year by 2020, corresponding to 20,000 patients with rare diseases together with their families, and 50,000 ‘high-priority patients’ with metastatic cancer or cancer refractory to treatment.
“For cancer patients, the idea is to use the sequence to find something that could be the starting point for an approved treatment or entry to a clinical trial with matched therapy. This means sequencing won’t be offered initially to patients with a poor performance status or liver or renal dysfunction, who wouldn’t be eligible for clinical trials,” says Christophe Le Tourneau, from the Institut Curie, Paris, who is involved in the Parisian Sequoia platform selected by the plan.
The platforms will be supported by two national centres for expert analysis to ensure consistency and provide access to molecular biology boards (consisting of biologists and pathologists), who will be on hand to provide advice on key decisions around prioritisation of which mutation to target first.The UK 100,000 Genomes Project
In the UK, the 100,000 Genomes Project was launched in 2013 with the intention of transforming molecular pathology and enabling WGS to become part of routine clinical care in the National Health Service (NHS). The initiative, encompassing cancer and rare diseases, is run by Genomics England, a company owned by the Department of Health and Social Care.
Thirteen NHS Genomic Medicine Centres (GMCs) have been established across England, located in major hospitals, which act as hubs, linked to more than 90 local recruiting hospitals. Initially recruitment was restricted to common tumour types (breast, colorectal, and lung), but it has since been extended to cover all cancers.
The project set the target goal of sequencing approximately 40,000 genomes in cancer; 17,000 samples have been submitted for analysis so far. Additional goals include providing data for scientific discovery and kick-starting development of the UK genomics industry.
After cancer samples are biopsied at local hospitals, tissue preparation, DNA extraction, and quantification take place within NHS GMCs to standardised protocols. DNA is then transferred to a Central National Biorepository, where they ensure that the right quantity and quality is sent for processing.
Processed files are sent back to Genomics England’s headquarters, who prepare reports for clinicians highlighting potentially ‘actionable’ genes that can be targeted by NHS-approved drugs and eligibility to UK trials. Reports additionally provide supplementary analysis of copy number variation, pan-genomic markers and mutational burden, which may be of value for predicting response to immunotherapies. The current aim is to return reports within a’ clinically meaningful’ timescale of 14 days.
Tumour boards have been established at each of the NHS GMCs, consisting of laboratory scientists, oncologists, pathologists and germline geneticists, to provide advice on individual patient treatment.
Getting governments to act
Europe, however, is not the US. The big challenge for initiating any change in healthcare across member states is that responsibility for health lies principally at the level of the national governments. “Perversely, while health is considered a national issue in Europe, research comes under the competence of the European Union, which leads to a lack of consistency,” says EORTC’s Golfinopoulos.
The European Cancer Patient Coalition believes patients could be an effective force to lobby their own governments, as politicians are more likely to listen to their concerns than to pressure from professional groups. But while patients will fight for access to therapies that could benefit them, the idea of fighting for tests to see whether or not they could benefit from a particular drug requires greater knowledge and understanding.
In November, which has been designated ‘Personalised Medicine Awareness month’, ECPC will be launching a major advocacy campaign to improve genomic literacy among the public and remove this stumbling block for integration. “At the moment we face the situation where many patients don’t know to ask for it and doctors don’t know to offer it,” says Makaroff.
“We’re calling on all our members to come together and demand harmonised access to biomarker testing across Europe. We want them to let policy makers in their countries know that precision medicine exists, and help them to appreciate that giving the right treatment to the right patient is something that will ultimately save lives and money,” she says.
There is also a move to encourage the Austrian Presidency of the European Council, which runs until the end of 2018, to take up the cause of equal access to oncology testing across Europe. “While we can’t hope to introduce health legislation across Europe, what would be helpful is to have a simple message coming from the European parliament that they support testing,” says the Cancer Drug Development Forum’s Zwierzina.
“What’s really concerning is that anyone in Europe can set up a testing service”
Whether such testing services are based on panel tests or whole genome sequencing, which requires a much higher level of investment (see box, opposite), is less important than ensuring that access to relevant, reliable testing is available.
Looking ahead, however, genomic information may turn out to have a valuable role to play as a prognostic indicator to predict the course of disease and need for follow-up, as well as in a prevention setting, where it could be used to spot tendencies towards developing a particular disease.
Eventually, personal genome sequences obtained at birth are likely to become integral to the patient’s electronic health record (see also, ‘Need a doctor? Send in your digital twin’).
But if European countries cannot cope with the current situation, it begs the question of how they will navigate the future explosion of information.