
As the European Commission issues its first call for proposals to set up European reference networks, Anna Wagstaff asks: how can these cross-border healthcare structures improve the quality of care received by the almost half a million Europeans who are diagnosed with a rare cancer each year.
Nobody wants to be told that their 15-year-old daughter has a cancer that cannot be removed without cutting out her entire stomach. But when the medical team – at one of the top children’s hospitals in the country – also tells you that they’ve never seen anything like it before and don’t know exactly how to treat it, that is a very lonely and frightening place to be.
That is certainly how Jayne Bressington felt six years ago. The surgeons, who had aborted an operation to remove the growth after seeing how far it had invaded the young teenager’s stomach, had taken an informed guess that it could be a gastro-intestinal stromal tumour (GIST) – a rare type of sarcoma, which is itself a rare cancer, and is most commonly found in 50- to 70-year-olds.
Tests revealed they were in the right area: it was a rare form of GIST, known as a paediatric-adolescent wild-type syndromic (PAWS) GIST – which was more a description than classification, being a GIST that occurs in young people and does not have either the KIT or PDGFRA mutation, which characterise 85% of all GISTs. So an extremely rare cancer.
The advice was to agree to the removal of her daughter’s stomach. Bleeding from the tumour was causing severe anaemia that could be controlled only through regular transfusions, and would eventually be life threatening. It had to come out.
Bressington was not keen. Like many people in similar situations, she turned to the Internet. She would have given anything at that point to have been directed to a PAWS-GIST centre of excellence in the UK, or indeed anywhere in Europe – somewhere that specialised in treating young people like her daughter, had experience caring for similar patients, and was engaged in research. But she found no such place.
Happily, thanks to a tip-off from one of the doctors who’d been doing some research of his own, Jayne and her daughter did find what they needed in the US. The only PAWS-GIST clinic in the world convened twice a year at the National Institutes of Health in Bethesda, Washington DC, flying in specialists from different disciplines from all over the country to consult with patients who found their way there.
Jayne Bressington brought two things back with her from that clinic. The first was the confidence to say “no” to surgery. The advice from “the most knowledgeable people in the world,” had been categorical: “Resist at all costs having your stomach removed. You have to find every way possible to stop the bleeding. You’ve got to grow, you need your nutrition, you need a stomach.” The second thing she brought home was a determination to see a similar clinic set up in the UK.
European Reference Networks
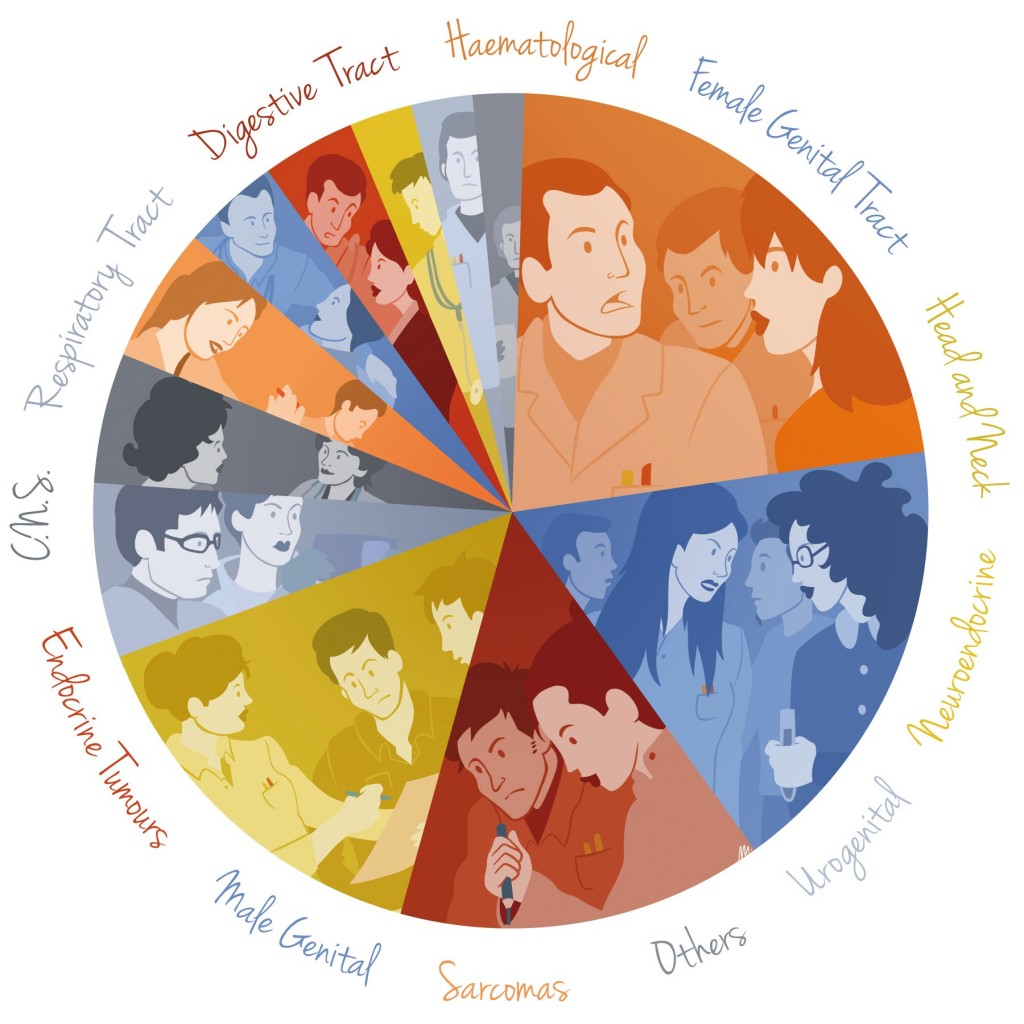
There are almost 200 different types of rare cancer (defined as fewer than 6 cases per year per 100,000 people), and every year, more than half a million people in Europe will be diagnosed with one (EJC 2011, 47:2493–2511).
Around 120,000 of these will be cancers that are seen in fewer than 1 person per 100,000. Many of those affected scour the internet, as Jayne Bressington did, to find doctors and centres with the expertise to give them the best possible chance of surviving with a good quality of life. Many will not find what they are looking for.
Their chances of finding a specialist centre may considerably improve, however, thanks to an EU policy promoting the setting up of European reference networks, which formed part of the 2011 cross-border healthcare directive. The idea is to harmonise and improve the standard of care available to patients with rare diseases across Europe by building networks that link designated centres of expertise within and between the member states.
How these networks will work in practice remains to be seen. The first call for proposals was issued by the European Commission in mid-March. As healthcare is beyond the competence of the European Commission, power to approve or reject proposals has been put in the hands of a ‘Board of Member States’, which will deliberate on the first round of proposals sometime after the summer deadline, and make its decision. Paolo Casali, chair of the campaigning group Rare Cancers Europe (rarecancerseurope.org), and a trail-blazer in rare cancer networking, is waiting with equal measures of hope and trepidation.
Hopes and fears
When it comes to networking to improve the care of people with rare cancers, no-one does it better than the paediatric oncologists. Every paediatric cancer is a rare cancer, and for decades this group of specialist clinicians have been collaborating on clinical trials to learn how to get the best possible results for their young patients.
In recent years, specialists in other forms of rare cancers have begun to follow their lead and have used EU funding to set up their own networking projects. Casali himself played a key role in setting up the Concatinet network, which linked teams in a number of European countries with expertise in diagnosing and treating more than 25 types of connective tissue cancers known as sarcomas.
Casali’s biggest hope for European reference networks is that they will dovetail with rare disease communities like his that are already organising themselves and their work – his biggest fear is that they won’t.
One serious concern is that the Board of Member States has failed to grasp how many people are affected by rare cancers. “Using a conservative definition, rare cancers are 20% of new cancer cases. Clearly they are at the heart of the field of oncology. This must be properly understood or the networks will fail,” Casali warns.
A consensus exercise carried out by Rare Cancers Europe succeeded in sorting almost 200 types of rare cancer into a minimum of 12 family groups, each with its own community of experts, reference institutions and patients. The signals coming from the Board of Member States, however, is that they are looking to keep the total number of rare cancers networks very low – maybe two or three.
This might mean a single network for paediatric cancers, as has already been set up in the form of a three-year pilot project (see box), and one for haematological cancers, possibly grouped with other rare diseases of the blood. The expectation seems to be that the entire spectrum of adult solid rare cancers would be taken care of by a single network, even though each involves different communities and institutions, requires different approaches to diagnosis and management, and the specialists in Europe are already working together within their specific communities.
Casali accepts that it might be possible to organise subnetworks within one big network, but argues that this would add an unnecessary and bureaucratic layer of complexity. Specialists in sarcomas already work with one another and constantly meet at conferences and other forums, as do people specialising in head and neck cancers or endocrine tumours, and so forth, he says, so it makes sense to set up reference networks that mirror this reality.
The other big concern for Casali is research. Linking care and research has become a mantra throughout the cancer community, but nowhere is this more important than for rare cancers, where the evidence base for diagnosis and management is sorely lacking, and the small size of patient populations makes it imperative to recruit every patient possible into trials, or at least ensure that the details from each patient’s history contributes to building up new knowledge.
When pressed on this issue at a meeting on European reference networks called by the European Commission last October, however, the Commission was very clear: the primary purpose of reference networks is to provide care – they are not intended for research.
But Casali argues that the two can and should go hand in hand: “Care can be well accomplished without giving up the goal of research.”
The heavy focus on care is reflected in the structure of the networks, where only healthcare institutions can join as designated centres of expertise. Professional bodies that develop clinical practice guidelines, such as ESMO, and research organisations such as the EORTC – which is currently setting up a rare cancers screening platform to improve access to trials – will probably be relegated to operating on the fringes of the networks.
“Why not acknowledge and build on the reality of the networking that the oncology community has already built over recent decades?” asks Casali, “rather than acting as if oncology networking in Europe is a blank slate.”
Making the networks work
Even in the worst case scenario, Casali recognises that the European reference networks will mark an important step forward, because centres joining the networks will be endorsed by governments. This means that patients will have somewhere in Europe to turn to that has been endorsed by its government, and is linked to a formal European network.
It also creates conditions for building networks within countries, and promoting policies on referral or shared care to ensure that the diagnosis and care of patients with rare cancers is handled by professionals with the greatest expertise, and not by the first doctor they encounter. “Clearly some health systems work better on rare cancers than others,” says Casali, “This could lead to a kind of harmonisation, because governments are involved.”
That said, the rare cancers community is not intending to sit idly by to see how these networks develop, says Casali. Rare Cancers Europe has been instrumental in getting agreement to set up a European Joint Action on Rare Cancers, “with the overarching aim of helping shape European reference networks in the best way possible for member states.”
The Joint Action is going to have to move pretty quickly, given that the networks have already been defined and the first call for proposals has been issued. However, there is a lot still to play for in how these networks will operate in practice.
Because the Joint Action group includes representatives from many member states, it should offer the chance to look at how European reference networks can meet varying needs and priorities in different countries.
Casali, for instance, based in Italy – population 60 million – sees European reference networks more in terms of “networks of [national] networks”. Italy records 2000 new sarcoma cases each year, so the role of its national hub will be to ensure that patients diagnosed anywhere in the country benefit from expert diagnostics and care planning, rather than discussing routine cases across borders.
Slovenia, by contrast, with its population of 2 million, can expect to see closer to 100 cases a year, spread between many different types of sarcoma, diagnosed at different stages and in patients with different needs and priorities. Slovenian sarcoma specialists may well value the opportunity to discuss cases with experts in other countries. They may be less interested, however, in building a national network, as care of complex or rare cases is largely concentrated in Ljubljana’s Institute of Oncology.
Tanja Čufer, Professor of Oncology at the University of Ljubljana, would like the Joint Action to raise the issue of access to clinical trials in other EU countries, which she sees as crucial for people with rare cancers, and is not covered by the reference networks’ remit. She points out that, “There are more and more small countries, and more and more rare cancers,” and says a solution must be found.
She gives as an example, ROS-positive lung cancer, which makes up just 1% of non-small-cell lung cancers. “There is no routine care, so these patients need access to clinical trials in larger countries, because we don’t have clinical trials for all these rare cancers in such a small country.”
Winning the argument on cross-border access to trials, she hopes, may be easier once you have accredited centres and European networks to make the case.
Patient advocacy groups have their own priorities. For Paulina Gmaj, who is active in the Polish sarcoma patient advocacy group Stowarzyszenie Pomocy Chorym na Mięsaki Sarcoma, having a government-designated centre of expertise is not the big issue. Poland does have an institution that acts as a reference centre – the problem is it has only one (for adult patients), serving a population of almost 40 million spread across a very large country. For her, the major obstacles include late diagnosis due to poor awareness among the public and GPs; lack of accurate information for patients and poor doctor–patient communication; and poverty, which limits access to best care. In Poland, she says, many people can’t afford to travel for appointments within the country, let alone across borders.
Gmaj believes that effective European networks could do a lot to address at least some of these needs. They could, for instance, develop patient friendly information for advocacy groups to disseminate (including information about clinical trials for those who can afford to pay). They could also give patients access to second opinions, and help harmonise standards of care.
In Belgium, the problem is almost the reverse. Véronique de Graeve, President of the NET & MEN advocacy group for patients with neuroendocrine tumours and multiple endocrine neoplasia, says that Belgium has several centres and professionals with expertise in NETs (less so for MENs), but that patients often don’t know where to find them. “Even general practitioners don’t really know where to refer their patients so the best care can be given,” she says, “because we still don’t have official national lists with experienced or recognised NET doctors or centres.” The government, she adds, is in the process of setting up a patients’ portal to provide relevant information to both patients and professionals.
de Graeve’s concerns are that the European reference network model, with its emphasis on centres of expertise, could lead to pressures for services to be more centralised than they need to be. “An isolated NET reference centre is not the way we see it in Belgium,” she says. “I prefer the ‘shared care’ between reference centres and peripheral hospitals… you need to respect what people are used to.”
The paediatric pilot
Reference networks are being been piloted in paediatric oncology. The three-year ExPO-r-Net project (European Expert Paediatric Oncology Reference Network for Diagnostics and Treatment – http://expornet.siope.comsbox.com/), was launched in 2014 to build a European Reference Network for Paediatric Oncology.
It has started:
tackling technical and legal (privacy and medical liability) issues involved in conducting cross-border tumour boards
identifying the types of patient who need a particular concentration of resources or expertise, where European networking could be most valuable
setting up a partnering scheme to improve access to high-quality healthcare in countries where that is not available due to low case volumes and/or lack of local resources – the emphasis is on moving information, not the patient, wherever possible.ExPO-r-Net involves 18 core partners and more than 50 collaborating professional partners (professionals, hospitals, institutes) from 17 countries, as well as parents and patients.
Room for manoeuvre?
There are, in short, plenty of views and opinions about how European reference networks should function. But will the rare cancers community really be able to influence how they develop in practice?
If the PAWS-GIST story in the UK is anything to go by, the answer is an unequivocal yes. On her return from the US, Bressington started her quest to found a similar specialist clinic in the UK with a Google search for “Dr+GIST”, which came up with 33 names in the UK. Together with a patient advocate from GIST Support UK, she wrote to them all, saying, “We’re in this terrible situation. Nobody knows what’s ailing our daughters, and there is no treatment. We want to set up a focus group in the UK.”
Eleven responded; one of them, Ramesh Balusu at Addenbrookes in Cambridge, saying he would be happy to lead the initiative. Four years of frenetic activity followed, raising funds, setting up a tissue bank, sorting out a registry and increasing the pool of patients from the three they started with to 70. They also set up a PAWS-GIST collaborative research initiative – a multidisciplinary team effort that aims to improve care and find innovative treatments for patients with this rare cancer.
If you Google PAWS-GIST from anywhere in the world now, you will find your way to the world’s second PAWS-GIST clinic (www.pawsgistclinic.org.uk), which convenes four times a year, has so far seen 40 patients, and is about to be written into the latest edition of the UK national guidelines for diagnosing and managing GIST. Patients across Europe get in touch, and specialists approach Balusu at conferences to talk about setting up something similar in their own countries. A few weeks ago, PAWS-GIST received its first requests for seed funding to kickstart two research projects – “A dream come true,” says Bressington.
So what would Bressington look for in a European reference network? “It would have to be able to help transform the situation from where we are now to where patients need to be,” she says, “ie a system that naturally facilitates research – a network of GIST registries, which includes mutational status; mutational testing as standard; a network of GIST tissue banks; a network of agreed specialist centres focusing on PAWS-GIST patients in collaboration with their local physician.”
It doesn’t sound quite what the Commission has in mind. But as we await the responses to the first call for proposals, there is still much to play for. With determined players like Bressington on the field, there may still be a chance to ensure that the reference networks provide what people with rare cancers really need.
The world’s second PAWS-GIST clinic. Jayne Bressington (far right), who was instrumental in making it happen, is pictured with (from right to left) Dochka Davidson (sarcoma specialist nurse), Richard Hardwick, (upper GI tract surgeon), Ramesh Bulusu (clinical oncologist, and clinical lead for the PAWS-GIST clinic), Palma Dileo (medical oncologist specialising in sarcoma) and Jason Bossert (formerly project manager).
European reference networks could help ensure patients with rare cancers like PAWS-GIST have a government-accredited reference centre somewhere in Europe they can turn to. But their impact on boosting research and spreading best practice will depend on how well they dovetail with the way rare cancers communities already work together.



