“There are doctors who do not inform the patients about [non-reimbursed options] because it is less stressful”.
“I explain to them that if they were treated in more developed countries, the treatment strategy would be different, and show them where they can find information about it”.
“When you have learned from a clinical trial or a congress that there is a new indication for treatment, there may be a lapse of time from the moment the treatment is approved at the European level until the treatment is reimbursed by the government. In that period of time we do not discuss those treatments with our patients because we, as a public hospital, cannot offer them. We do not speak about them because the great majority of the patients cannot afford that treatment”.
Clinicians have a duty to discuss all the options with their patients, to help them decide what’s best for them. But are ‘options’ that are unaffordable and not reimbursed truly options?
Does telling patients about treatments that could benefit them, but they cannot access, help efforts to reach the right decision or just confuse the issue and add to the patient’s distress?
Should doctors wait for patients to ask about therapies that are only available to those who can pay?
Should you selectively mention non-reimbursed options, depending on your judgement of the value to the patient and whether they might have the resources to consider paying out-of-pocket?
Is it never right, under any circumstances, not to tell a patient about a therapy that might benefit them?
And how do you conduct the conversation with a cancer patient, when you know that choosing a particular option may have severe financial consequences for them and their family?
Cancer World asked alumni of the European School of Oncology to tell us about how they handle these conversations, about the principles that guide their approach, and about any guidelines or laws that may affect what options they discuss.
We contacted medical/clinical oncologists, radiotherapists and surgeons – these conversations are not only about unaffordable medical options, but also diagnostic tests and imaging, specialist surgery, better targeted radiotherapy techniques… and even timely access to standard radiotherapy.
And we contacted readers from across Europe. The number of therapeutic options not reimbursed, and their potential value to patients, is certainly higher in the less wealthy parts of Europe. But it is a challenge for doctors and their patients in high-income countries as well, due to delays between regulatory approval and a decision on reimbursement, as well as the cost and marginal benefit of some new treatments or indications. More than seven in ten respondents from the low-income countries said the issue arose ‘very often’ or ‘quite often’, compared with three in ten from the most wealthy countries.
More than 100 cancer professionals (78 medical/clinical oncologists, 17 radiotherapists and 26 cancer surgeons and two dermato-oncologists) responded to the survey. Details of which countries are represented are given in the box on p 60, along with details of how we categorised them into high-, middle- and low-income groups. (The categories high, middle and low are relative to the European, not global, context.)
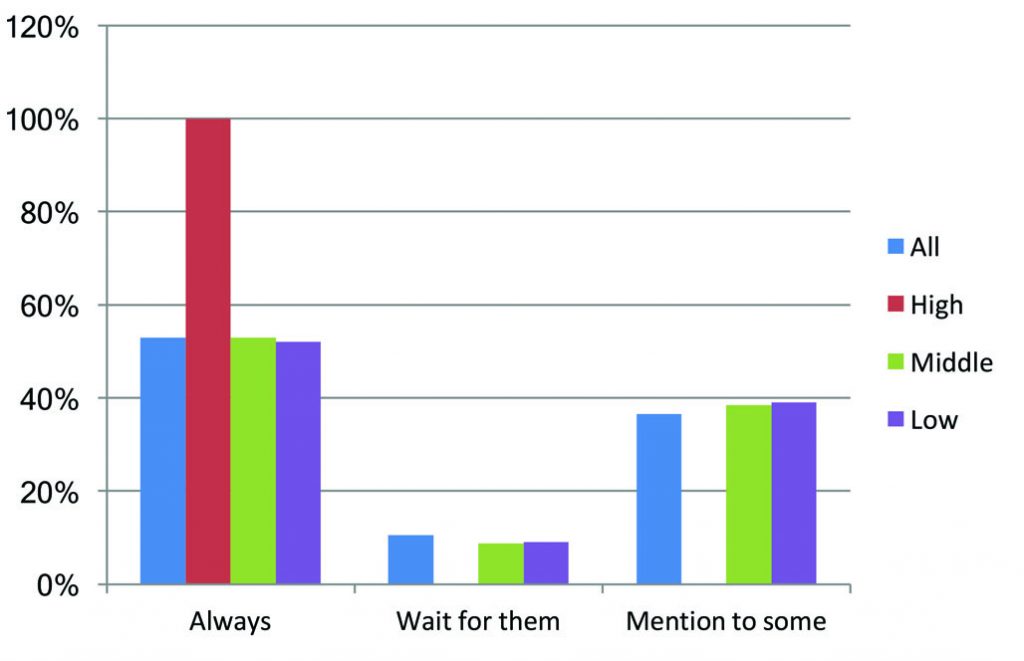
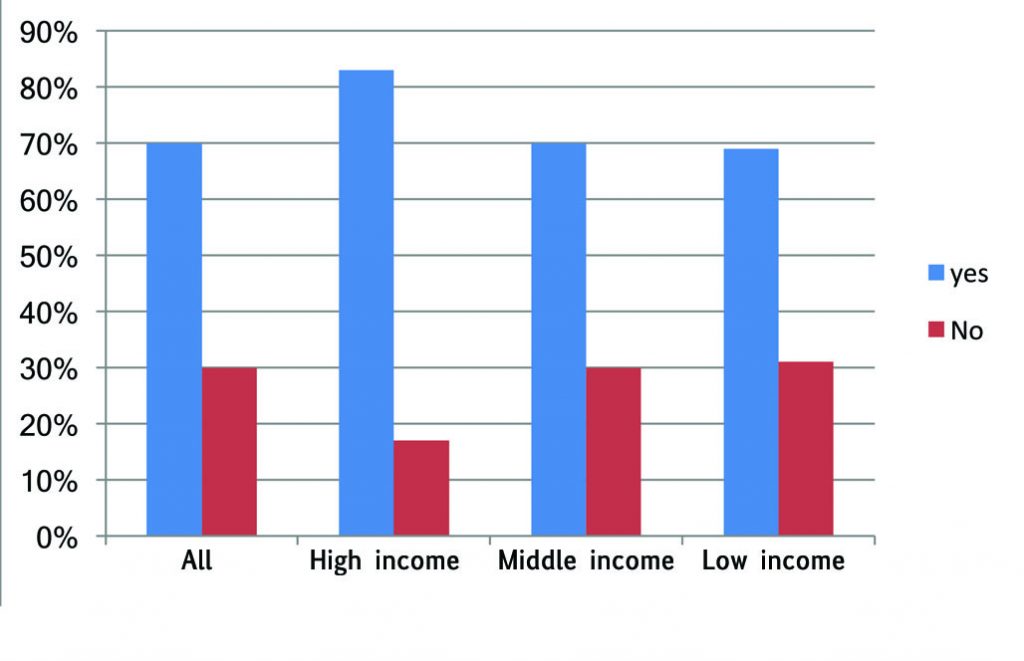
Do you mention it?
Just over half of all respondents said that, if there is an approved therapy that they know might benefit their patient, but they think the patient cannot access, they will always mention it. Almost four in ten said they mention it to some and not others. Only one in ten said they never talk about it unless the patient takes the initiative to ask about it.
There is a notable difference between the richest countries and the rest. Every respondent from the countries with highest income level said they would ‘always’ mention it. This may, in part, reflect that it is easier to talk to patients about non-reimbursed options in settings where the great majority of therapies that can really make a difference are in fact reimbursed.
Do you mention it?
Responses by country per capita GDP level
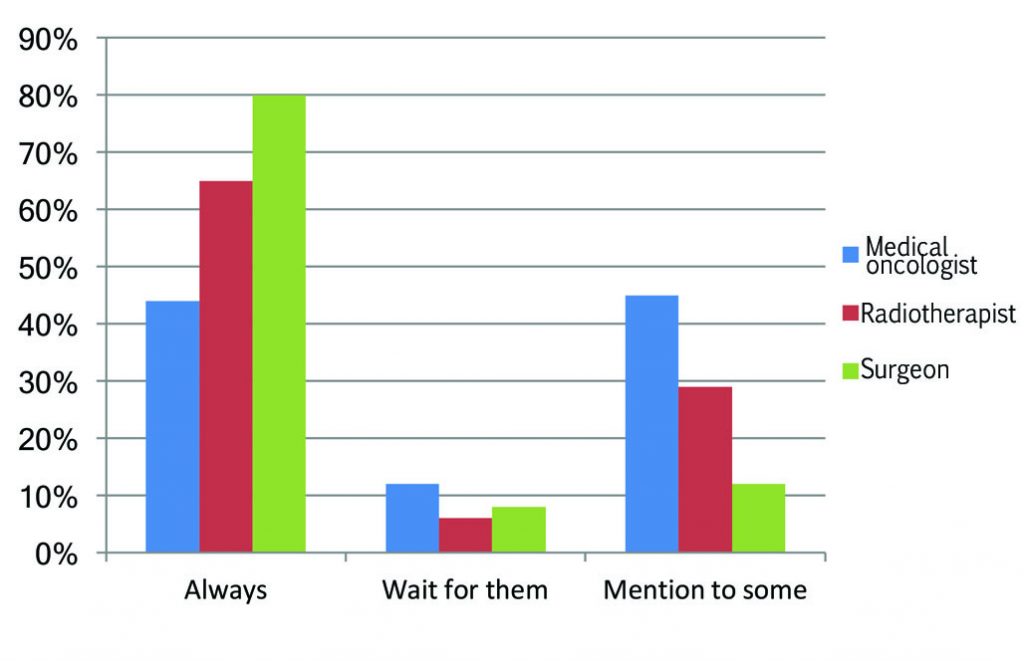
Responses by discipline
“The positive effect of most [of these] therapies is mostly minimal, so the loss of not having a certain treatment is limited,” was one comment.
A slim majority of the respondents from low- and middle-income countries in Europe also said they mention all therapeutic options to all their patients, while a substantial minority mention it only to some.
Some respondents, mainly in middle-income countries, said they try to help patients get funding, for instance, from charities and ‘medical need’ programmes, or find somewhere where a drug that has been approved but not yet authorised for reimbursement may be accessible via a clinical trial.
They are also aware of the added responsibility of not overstating the potential benefits when considering treatments that could have such a lasting impact on the finances of the patient and their family. “I approach it with caution, not to give false hope to patients.” “In any case I submit the proposal to more experienced colleagues or to my multidisciplinary group.”
Some respondents in low-income countries stressed the need to be transparent about the standard of care in international guidelines. “I explain to them that if they were treated in more developed countries, the treatment strategy would be different, and show them where they can find information about it,” said one.
“I think that I have to inform them fully of the options and prices. The most difficult part is to explain why the price of medicine is so high – this is completely incomprehensible for the patients,” said another.
Other comments indicate a tendency to stick to what seem realistic options for the patients in front of them, such as: “Most of my patients in a public hospital can’t afford to pay for expensive treatments,” or “We live in low-income country.”
There is also a notable difference between disciplines, with medical oncologists being the least likely to always mention it, and four times more likely than surgeons to be selective about to which patients they mention approved options that are unaffordable and not reimbursed.
This may reflect the fact that they see many more patients with incurable cancers. As one respondent noted, a toxic drug that could increase the chances of a cure given as an adjuvant or neoadjuvant in a medium- or high-risk curative setting could offer only marginal advantage to a patient with incurable disease. Medical oncologists may feel it therefore makes sense to be selective about who they mention the option to, particularly given the high cost of these therapies, which makes private payment out of the question for most people.
Rules and Guidelines
In many cases the choice of what to tell patients is influenced by laws and guidelines operating at a national or local level. These may be designed to promote ‘individual patient choice’, or to standardise treatment offered within a public healthcare system.
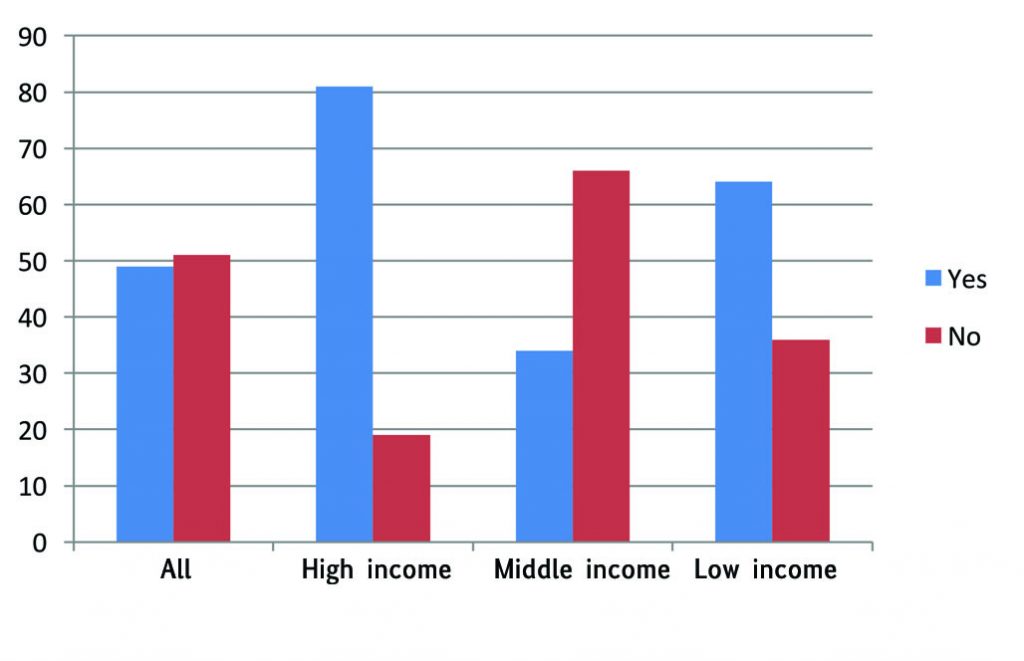
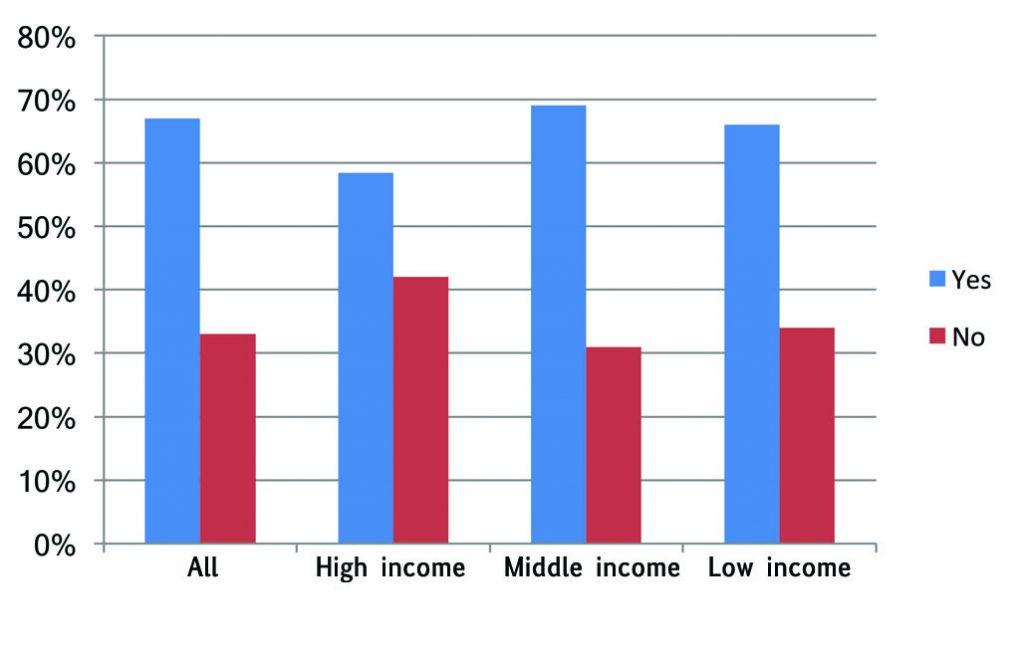
Responses to the survey question: “Are there rules governing what therapeutic options doctors are obliged to mention?” may not be very reliable, as respondents from the same country did not always agree. However, taken as a whole, there does seem to be a signal that the wealthier countries and the poorest have more regulations and guidance regarding which options should be discussed than middle-income countries. “In France we cannot ask patients to pay for their treatment,” said one respondent. Another, from Spain, commented, “In our system, any mention of an expensive therapy not covered by the system is out of the question. Depending on the potential benefits for the patients, there may be a frustrating situation for me.”
Rules and guidelines?
Workable for oncologists?
Best interests of patients?
Respondents also see an upside to having such guidelines, however. A clear majority feel that on balance the rules and guidelines are workable for oncologists, and a slim majority feel that on balance they also work in the best interests of patients. Comments referred to the possibility of bias in the way oncologists present information about potential risks and benefits, either due to their own prejudices or financial vested interests. One respondent pointed out that having guidelines on what to mention, “makes our job easier,” and another noted it could be “especially useful for less experienced oncologists. They make it possible to give information in the same way. Less confusing for the patient.”
A stressful conversation
Whatever the guidelines for discussing options may say, there is a widespread feeling that, too often, oncologists are being left to take the strain of the gap between what is approved and what patients can access.
This problem is most starkly illustrated, perhaps, in one Balkan country, where according to one survey respondent, oncologists are sometimes obliged to take personal financial responsibility for costs incurred from prescribing certain anti-cancer drugs.
“In some oncological institutions, physicians are obliged to sign a paper in which they state that they will prescribe only medicines that are reimbursed, or they will pay out of pocket the treatment which is not reimbursed, since the health insurance fund will charge the hospital for this treatment,” he says. “So, most physicians don’t mention the standard of care treatment based on European guidelines to their patients if this is not reimbursed, unless this is a patient they personally know and they can trust.”
The situation arises he explains because, while in principle healthcare is a universal constitutional right, in practice hospitals cannot afford all the recommended treatments. Oncologists, however, do not want to adapt European guidelines in a way that would exclude treatments that can significantly prolong patients’ life, improve their quality of life and even induce long remissions. So the hospitals end up putting pressure on individual oncologists to restrict their prescribing.
Survey respondents: where they practice
The survey was sent to more than 3000 alumni of the European School of Oncology who define their discipline as Medical Oncology, Clinical Oncology, Radiation Oncology or Surgery and who practice in a European country. We received 113 responses (78 medical/clinical oncologists, 17 radiotherapists, 26 cancer surgeons and two dermato-oncologists). Respondents came from the following 32 countries: Albania, Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Denmark, France, Georgia, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Montenegro, Netherlands, North Macedonia, Poland, Portugal, Romania, Russian Federation, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey, Ukraine, UK. Countries were grouped into high-, middle- or low-income using the 2018 International Monetary Fund data for GDP per capita at purchasing power parity, using the IMF brackets of >$50,000, $30‒50,000 and >$30,000.
Obliging oncologists to take personal legal responsibility like this for the cost of non-reimbursed prescriptions is probably an exception. But responsibility for talking to patients about therapies that could help them but are unaffordable is in any case a difficult conversation to have. This is another reason doctors often choose not to mention it, says another practitioner from the Balkan region, who treats patients with advanced melanoma. “I try to explain to every patient what are the options and that there is no reimbursement,” she says, “[but] there are doctors who do not inform the patients about this possibility, because it is less stressful than to explain every day what is the best option for them.
“The problem is this space between new innovations in medicine that are developing very fast, and our system that is not able to adapt to it, to negotiate for example prices with companies. And then the doctors are left to deal with it, to wait for the reimbursement, and patients – they are in the worst situation, of course – and that is really a very large frustration… As a result, many doctors are leaving the country.”
Ironically, that frustration may be even higher in many wealthier countries, with stronger public healthcare systems, where people have higher expectations about their right to access therapies approved for their indication – and again it is the oncologists who have to handle those conversations. Sometimes the reason is because the authorities decide that a new therapy or a new indication for its use represents poor value for money. Often, however, the problem is the delay between being approved for market and getting a decision on reimbursement. When the therapy is seen as quite effective, those conversations can be seriously stressful for all parties.
“This happened a lot at the beginning of immunotherapy,” said one oncologist from Spain, where 95% of all cancer patients are treated within the public system. He remembers discussing options with a patient who wanted to be treated with immunotherapy before a decision had been taken to reimburse it. He had to explain that, even if the patient were to pay for the therapy, it is not possible to administer it within the public health system.
What will I say?
John Crown, leading Irish breast oncologist and political cancer activist, responds on Twitter to the news that “For the first time we have differential public versus private access for cancer drugs”, following the approval by Ireland’s largest health insurer to cover costs of certain immunotherapies for additional indications.
During the time period between approval and reimbursement, he added, “we do not discuss those treatments with our patients, because we, as a public hospital, cannot offer them. In fact, we do not speak about them because the great majority of the patients cannot afford that treatment.”
In Italy a recent change to the law means doctors can now prescribe and administer drugs that are not reimbursed by the public health system. Oncologists are now likely to feel more responsibility to ensure that such an option is discussed with patients, even though in practice, the high cost poses an insuperable barrier for most.
The issue arises at least once a week, according to one genitourinary oncologist, who has seen his own patients with metastatic bladder cancer unable to get the PD-1 inhibitor, pembrolizumab during the gap between approval for that indication and a decision on reimbursement.
“I very seldom found patients who were willing to pay for a cancer drug,” he says. “The only recent case that I can recall is a colleague’s patient who was diagnosed with ROS-1 rearranged lung adenocarcinoma but could not receive crizotinib through our health system because it was not yet approved by AIFA [the Italian Medicines Agency] for that indication. He paid for five months of therapy, after which crizotinib was eventually approved. He told me that he would have been able to pay for not longer than one year of therapy.”
In rare cases, he adds, it may be possible to find somewhere the drug is being trialled, for instance in a combination therapy. But even this solution doesn’t work for most patients, he says, because they can’t face the travel or simply prefer to be treated in a familiar environment where they know the physician, nurses and other caregivers.
In some eastern European countries, discussing out-of-pocket options is common practice. “Frequently, we have a situation that involves supplementary costs for patients without talking about the treatment,” says one Romanian oncologist. “For instance, in the case of an investigation like PET-CT, for covering it by National Health Insurance House, a commission gathers once a month and approves the cases that need the investigation. There are patients who do not want to wait for a month and prefer to pay around €1,000 to obtain this investigation faster.”
Doing the right thing
What is the right thing to do? Should doctors always mention every ‘option’ to every patient, even when they are certain that the information does not add to their options in any meaningful way, yet could add to their stress by focusing on what cannot be achieved. Should doctors ever make assumptions about what is or is not relevant information for their patients?
There are no simple answers, says Giovanni Boniolo, Professor of Philosophy of Science and Medical Humanities at the University of Ferrara, Italy. “In principle, the best strategy should be to tell all the possibilities in a truthful way. Of course, this kind of communication should be made with great care. But there could be instances in which the omission of certain information could be beneficial.”
He feels oncologists should get more support and training in how to handle difficult conversations like these in a way that balances principles of truth telling and transparency with a responsibility to avoid adding unnecessarily to the distress of patients.
“Doctors are not trained to face ethical questions,” he says, “they rely on ‘common sense’.” He would like to see ethical issues and ethical reasoning being taught as part of medical training. Ensuring doctors feel better equipped to handle these difficult conversations could not only benefit patients and their families, he adds, but could lessen the stress felt by doctors, which, he says, “is one of the main elements leading to burnout syndrome.”
Join the conversation
Cancer World’s series of ‘Getting personal’ articles aims to offer a clinicians’ perspective on ethical dilemmas that are common on consultations with cancer patients. Thanks to all the respondents who took time to answer our survey questions and do follow-up interviews for this article. If you would like to contribute information about your own experiences handling conversations with patients about options they cannot afford, please go to the online article at bit.ly/CW86-WhatDoYouSay and leave a comment.




Leave a Reply