Could the new generation of antibodydrug conjugates herald a move away from conventional untargeted chemotherapy? Much will depend on how far and in whom their added benefit can justify the high cost of these technologically sophisticated drugs.
 After an apparent lull in progress of more than a decade, in the last two years, two antibody–drug conjugates (ADCs) have been approved. ADCs combine an antibody designed to target cancer cells, with a linker molecule connected to a highly potent cell killing toxin. They can therefore deliver anticancer agents directly to tumour cells, limiting the exposure of healthy tissue to the toxic drug, with the view to providing more successful treatments with fewer side effects.
After an apparent lull in progress of more than a decade, in the last two years, two antibody–drug conjugates (ADCs) have been approved. ADCs combine an antibody designed to target cancer cells, with a linker molecule connected to a highly potent cell killing toxin. They can therefore deliver anticancer agents directly to tumour cells, limiting the exposure of healthy tissue to the toxic drug, with the view to providing more successful treatments with fewer side effects.
Today, around 70 ADC clinical trials are underway for cancers including the lymphomas, breast, colorectal, kidney and lung. So does this current renaissance in ADCs finally herald the arrival of a new generation of highly effective but less toxic cancer treatments?
The development of ADCs has not been without false starts. The idea dates back to 1897 when German Nobel laureate and founder of chemotherapy, Paul Ehrlich, noted “antibodies are in a way magic bullets that identify their target themselves without harming the organism.” He envisioned that, by attaching toxins to them, such a therapy could selectively kill microbes or cancer cells. The first ADC to receive regulatory approval was 15 years ago. Mylotarg (gemtuzumab ozogamicin) received accelerated approval in the US for use in patients aged over 60 with relapsed acute myelogenous leukaemia. But in 2010, Pfizer withdrew the drug from the US market after a follow-up trial showed no improvement in clinical benefit and a greater number of deaths in those who received it, compared to those receiving chemo-therapy alone.
Although there are ongoing European trials using a lower dosage, Mylotarg’s initial failure illustrates some of the fundamental problems with the first generation of ADCs. ADC drug payloads are more toxic than most conventional chemotherapy drugs, so if targeting is not accurate, there is the potential for more, rather than less, damage to healthy cells. With Mylotarg, the suggestion was that its target, the cell-surface protein CD33, was not as selective for tumour cells as first thought, and there were also problems with early breakage of the linker between the antibody and drug, allowing the toxic payload to be released before reaching its target.
No other ADCs made it to market between 2000 and 2011, but after 10 years of further research a second generation of ADCs is now emerging. The first of the two currently licensed ADCs is Seattle Genetics’ Adcetris (brentuximab vedotin), approved in 2011 in the US and 2012 in Europe for relapsed Hodgkin lymphoma and relapsed anaplastic large cell lymphoma. Composed of the antibody brentuximab linked to the cancer toxin monomethyl auristatin E (MMAE or vedotin, when conjugated), it targets the cell surface antigen CD30. The second, Roche’s Kadcyla (trastuzumab emtansine), was approved in the US and Europe in 2013 for advanced HER2-positive breast cancer. It uniquely combines two active components: the HER2 targeting antibody Herceptin (trastuzumab) and the toxin mertansine. Kadcyla can add six months to the survival of patients with metastatic breast cancer (NEJM 2012 367:1783–91), whilst Adcetris is showing convincing patient survival data (Abstracts 3689, 3701, ASH 2012).
Ironing out the glitches
Early ADCs needed improvement in all areas including the antibody. David Thurston, Professor of Drug Discovery at Kings College London, explains that in the late 1980s, the first ADCs used mouse antibodies, but they didn’t work well because, “patients reacted significantly to the mouse antibody and the body got rid of them through excretion as quickly as possible.” Then came the creation of hybrid mouse–human antibodies and, finally, fully human monoclonal antibodies, produced using immune cells cloned from transgenic mice.
For successful ADCs the antibody target needs to be unique to cancer cells to avoid targeting healthy cells, and the level of expression needs to be high, at least 100,000 per cell to ensure cell death. The ideal antigen is internalised into the cell, along with the ADC. So far, the ideal antigen expression has been found more often in haematological cancers, but ADCs are presently in the pipeline for at least 24 different antigen targets in a variety of cancers.
According to Thurston, it is the toxic small-molecule drug payload that is the trickiest part of an ADC to get right. “You have got to deliver a drug that will kill the tumour cells effectively, so all the payloads that have been used so far – and there aren’t many – are all highly cytotoxic,” he says. The agents used can be over 100 times more potent than traditional chemotherapy drugs because, even with their high selectivity, only a small percentage can be expected to reach the tumour – one estimate is around 1.5% of the administered dose (Clin Cancer Res 2011, 17:6389–97).
Improvements to the stability and versatility of linkers is also a major advance. The first ADCs were created by directly connecting the toxin molecule to the antibody using a coupling agent, but this did not provide enough stability. Current technologies use a linker molecule, usually a simple peptide, connected most frequently via antibody amino acids. “In most cases the whole complex of the antibody, linker and payload is internalised,” says Thurston. “It goes inside the cell and then proteases just chew up the simple peptidic linker and release the drug.”
Another issue with early ADCs was the lack of uniformity in the number of attached drug molecules. Too few, and the ADC does not carry a large enough dose, too many and the conjugate becomes unstable, and may block the antibody binding site or reduce the conjugate’s half-life in circulation, so reducing target exposure time. The goal is to produce homogeneous conjugates, in most cases with three or four drug molecules per antibody. A solution to uniform drug loading is site-specific conjugation. Two of the major forces in ADC technology, Seattle Genetics and Genentech, have developed platforms that do this. They have engineered antibodies with substituted cysteines that are able to conjugate drugs in specific positions.
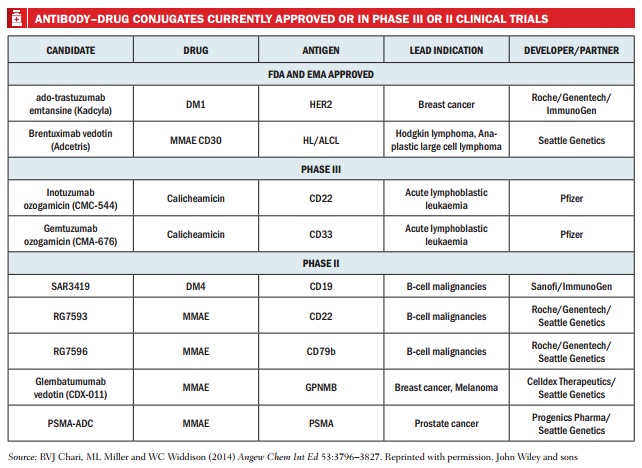 Second-generation ADCs
Second-generation ADCs
The renewed potential of ADCs is well illustrated from the results achieved with Adcetris. Adam Gibb, Clinical Research Fellow at the Christie Hospital in Manchester, UK, was part of the team carrying out the first trials outside the US in 2010–2011. Their study hit the media spotlight last year with the story of 47-year-old Ian Brooks, who received Adcetris after suffering a relapse of anaplastic large cell lymphoma.
Gibb describes the treatment as “spectacularly successful”, and says “it chewed through the disease in a matter of days” and the patient was in complete remission in 12 weeks. The EMA granted the drug conditional approval on the basis of evidence from this multicentre phase II open clinical trial. Brooks was one of 58 patients with relapsed anaplastic large cell lymphoma participating in the study, more than half of whom (57%) achieved complete remission, with a median duration of 13.2 months (JCO 2012, 30:2190–96). Among 102 patients who were treated with the drug for relapsed or refractory Hodgkin lymphoma, one-third (34%) achieved complete remission, with a median duration of response for those in remission of 20.5 months (JCO 2012, 30:2183–89).
The Christie trial proved particularly useful as a bridge to stem cell transplants by providing patients who had already undergone multiple relapses with high-quality remission. “A good few patients were satisfactorily salvaged through to being transplanted – that is really where the excitement with brentuximab is coming from,” says Gibb. As a single agent brentuximab vedotin is still described as ‘palliative’, rather than ‘curative’, but Gibb says that from the first Hodgkin lymphoma phase II trial, which took place in 2009–2010, 10–15% of patients are still alive. The drug is in ongoing trials for a wider range of uses, including two randomised phase III trials assessing brentuximab vedotin as a first line therapy in Hodgkin lymphoma and mature T-cell lymphomas which express the CD30 antigen that brentuximab targets.
“A good few patients were salvaged through to being transplanted – that is where the excitement is coming from”
Brentuximab vedotin also illustrates the advantages of reduced side effects the ADCs can offer. It is by no means free from adverse effects, which include fatigue, nausea, infection and critically neuropathy, which Gibb says is the side effect that caused many of his patients to “throw in the towel” after an average of 11 of a possible 16 cycles. It is, however, “a much better tolerated agent than the type of chemotherapy it is contrasted against in these settings,” he says. The off-target effects occur due to ‘bystander’ effects to nearby cells, but any healthy cells that express the antigen targeted by the ADCs are vulnerable. The CD30 antigen targeted by brentuximab vedotin is also expressed on activated lymphocytes. The washout from the dead tumour cells also presents a significant source of toxicity. But, in general, the targeted approach of the present generation of ADCs certainly promises a significantly gentler form of chemotherapy for the future.
Beyond the blood cancers
Of the ADCs currently in clinical trials, a higher proportion tackle haematological than solid cancers. This is largely because they typically express homogeneous and more unique antigens, making them easier to accurately target. But there can also be problems getting ADCs to penetrate into solid tumours. Biotechnology company Mersana Therapeutics is now developing a conjugation technology that could provide an answer to tackling harder-to-reach solid tumours. The company was spun out of Massachusetts General Hospital ten years ago to develop a biodegradable, well-tolerated polymer it calls ‘fleximer’. Their technology allows an increase in the ADCs’ toxic payload by linking many more drug molecules to the soluble, polyvalent polymer backbone, which is then attached to the antibody. Mersana CSO Timothy Lowinger explains “…we can take molecules of the auristatin class and we can attach 20 of them and still have excellent properties, and if you are delivering 20 drugs per antibody instead of three or four, you have much more efficient delivery.”
Mersana’s third-generation, fleximer-based ADCs could also allow targeting of tumours with lower antigen expression levels. The company has demonstrated this using HER2-expressing tumour models that express 50,000 antigens rather than the 500,000 commonly found in the patient population. According to Lowinger, “When we use the same antibody as Kadcyla, but attach with our technology 20 drugs, we can see that even low-expressing tumours are now highly susceptible, so that one can get completely tumour-free survivors in those same models that are completely non-responsive to Kadcyla.” This proof of principle clearly shows the potential for future ADC technologies.
Innovations are also underway in targeting. Moving away from large immunoglobins towards something smaller could provide better penetration into solid tumours, and a variety of approaches are being developed to achieve this. Improving the specificity of targeting tumour cells is another area of major interest. Engineered bispecific monoclonal antibodies (BsMAbs) – artificial antibodies composed of fragments from two different antibodies – make it possible to target two different antigens on the same tumour. Another strategy is to use BsMAbs that recognise antigens on a tumour cell and also activate the patient’s own T-lymphocytes, which can then destroy the tumour cell. This strategy is already being used with antibody therapies such as TRION Pharma’s Removab (catumaxomab), the first bispecific to receive European approval, for treating malignant ascites in patients with metastasising cancer and Amgen’s Blincyto (blinatumomab), which last December became the first bispecific antibody to be approved by the FDA, for use in patients with relapsed or refractory B-cell precursor acute lymphoblastic leukaemia.
ADCs are sure to play a big part in the future of cancer therapeutics. As ADC development expands to target more tumours, Mersana CSO Lowinger suggests “there may be the ability to move away from conventional untargeted chemotherapy completely.”
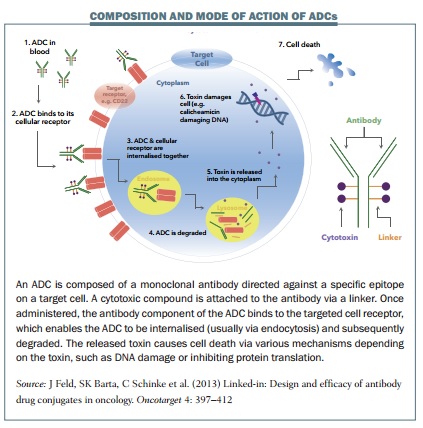 At present, Adam Gibb thinks, with cancers such as Hodgkin lymphoma, the key will be learning how to identify the 10–30% of patients who will relapse following conventional therapy, and would benefit from a targeted approach early in their disease course. ADCs, he says, may be better able to destroy slower growing cancer cells that are more resistant to chemotherapy, and most likely to be responsible for relapse and refractoriness.
At present, Adam Gibb thinks, with cancers such as Hodgkin lymphoma, the key will be learning how to identify the 10–30% of patients who will relapse following conventional therapy, and would benefit from a targeted approach early in their disease course. ADCs, he says, may be better able to destroy slower growing cancer cells that are more resistant to chemotherapy, and most likely to be responsible for relapse and refractoriness.
Costs and benefits
But the elephant in the room is the cost of these new drugs, which are expensive to develop. The number of ADCs gaining regulatory approval is likely to accelerate, with over 100 now in the pipeline. There have already been cost issues with Roche’s Kadcyla. It has been approved for reimbursement in France and a number of other European countries, but the UK’s NICE ruled that Kadcyla, with a full list price of £90,000 per patient, was too expensive for NHS use (in some cases it can be prescribed via the UK’s Cancer Drug Fund). Amgen’s Blincyto, meanwhile, represents a new record for cost of cancer treatment in the US, with a price tag of $178,000 per patient.
Richard Sullivan, director of the Institute of Cancer Policy at Kings College London and former clinical director of Cancer Research UK, says: “The question you have to ask is: are antibody–drug conjugates going to be clinically meaningful? – I suspect a lot of them will not.” He argues small incremental improvements from trial data may not translate into decent improvement in clinical outcomes, and these drugs are likely to fail or sit very near the bottom of economic benefit assessments. Adam Gibb suggests that more of a case can be made for an ADC such as Adcetris (which is also funded in the UK through the Cancer Drug Fund), which benefits a small group of mainly young patients (around two hundred a year in the UK), who relapse after chemotherapy and stem cell transplants. It is still an expensive drug, says Gibb, but “we are looking at being able to have a third bite at the potentially curative cherry.” He adds that the costs of the drug are still less than those associated with the donor stem-cell transplants given to this group of patients.
“We are looking at being able to have a third bite at the potentially curative cherry”
Problems with the high price of the branded drugs could also be compounded by potential problems surrounding development of ADC biosimilars – approved copies. Due to their molecular complexity and reliance on an originally cloned antibody, there are concerns that it may not be possible to produce copies without going through the entire development process again, “This could essentially kill any form of generic…so this is a double whammy,” says Sullivan, as without competing generics, prices are likely to stay high.
The arriving wave of ADCs is illustrating a wider issue, says Sullivan: “They are just one technology amongst a massive tsunami that is hitting healthcare.” He argues that there is a growing divergence and disconnection between the pharmaceutical industry’s business model, public expectations, and what in reality is affordable for Europe’s healthcare systems, which is leading to massive inequalities and irrational prescribing. He expects other European countries will move in the UK direction: “It would not surprise me if people get much much tougher over the next two to three years about what drugs are prescribed,” he says, adding that pharmaceutical companies will need to start engaging in “fair pricing”, particularly with medicines that provide relatively small incremental advances in health outcomes.
As Sullivan put it, ADCs give us “the beautiful science versus the messy dirty reality of socio-economics”. On the scientific and clinical side, after a decade of development, antibody–drug conjugates now promise a new generation of targeted chemotherapies that may be able to tackle relapsed and refractory cancers, untreatable by conventional means. While not free of side effects, these new drugs do promise a milder, more tolerable form of therapy. But if ADCs are going to benefit the widest possible group of patients, a rational re-think of how we pay for them will need to take place.
Cancer World journalists’ grant scheme
This article originated in a proposal submitted by the author for consideration for a Cancer World journalists’ grant, a scheme set up to encourage journalists working in print, broadcast or online mass media to tackle more complex, multi-source, analytical articles that explore systemic issues that have an impact on patients. Further information about the grant can be found at https://archive.cancerworld.net/media/cancerworld-journalist-grant/