Skin rash and itchy skin are known to be common side-effects in patients treated with EGFR and tyrosine kinase inhibitors, and can be distressing, particularly when severe. A growing understanding of why this happens is leading to new ways of managing the problem
 This e-oncoreview was sponsored by Helsinn
This e-oncoreview was sponsored by Helsinn
Pruritus, more commonly known as itchy skin, is an increasingly important issue in cancer, as a growing number of anti-cancer drugs can induce this reaction. Rash and pruritus are very common with EGFR inhibitor- and tyrosine kinase inhibitor- (TKI-) based treatments, occurring in around 10–80% of patients. The rash typically resembles acne or occurs as red papulopustules. It is dose-dependent and can affect all areas of the face and body, typically peaking between two and four weeks after the start of treatment. Bacterial, viral and fungal infections are all potential complications of skin toxicities with EGFR and tyrosine kinase inhibitors.
Skin toxicity is graded from mild to severe. In mild skin toxicity, lesions are generally localised. Lesions are generalised in moderate skin toxicity, but the symptoms are mild. Patients with severe skin toxicity have generalised lesions and very severe symptoms. Severe pruritus and skin rash can have a serious negative impact on quality of life in these patients.
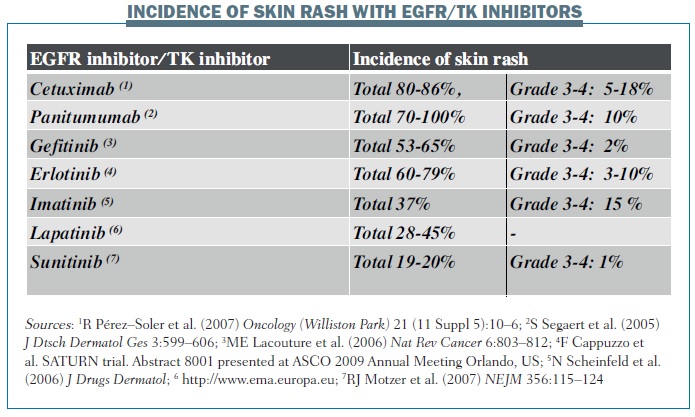
The incidence of skin rash varies between different biological therapies (see below). For example, the incidence of grade 3–4 skin rash with cetuximab is 5–18%, while it is 10% with panitumumab and 3–10% with erlotinib. The incidence of pruritus, as recorded in pivotal phase III trials, also varies between different EGFR and tyrosine kinase inhibitors – from 4–16% with cetuximab to a very high incidence of 57% with panitumumab. However, I think that the most important TKI for inducing pruritus is erlotinib.
 In a very interesting meta-analysis published last year, Mario Lacouture’s group demonstrated that all grades of pruritus are significantly increased with targeted cancer therapies compared with placebo (J Am Acad Dermatol 2013, 69:708–720). This meta-analysis demonstrates that patients treated with TKIs or EGFR therapies experience pruritus in their daily lives,
In a very interesting meta-analysis published last year, Mario Lacouture’s group demonstrated that all grades of pruritus are significantly increased with targeted cancer therapies compared with placebo (J Am Acad Dermatol 2013, 69:708–720). This meta-analysis demonstrates that patients treated with TKIs or EGFR therapies experience pruritus in their daily lives,
Pathophysiology of pruritus with targeted therapies
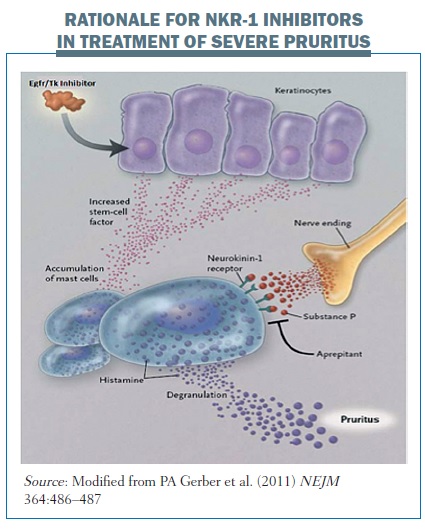
We know that substance P is one of the major neuromediators of pruritus. It binds to neurokinin receptors (NKR) 1, 2 and 3, but mainly to NKR-1, and represents a prominent activator of mast cells. NKR-1 is a G protein-coupled receptor localised not only in mast cells but also in the central and peripheral nervous system. It is also expressed by inflammatory cells. Substance P activates mast cells through NKR-1 and causes the release of pruritogens.
Biologic therapy with EGFR/TK inhibitors induces the secretion of stem cell factors and the subsequent accumulation of dermal mast cells in the skin of patients with biologic therapy-induced rash (see below). These stem cell factors activate mast cells and activate substance P to act on neurokinin-1 receptors expressed by mast cells. This releases pruritogens, which induce pruritus. Using an inhibitor of the neurokinin-1 receptor will inhibit the action of substance P and so block the release of pruritogens by mast cells.
 Aprepitant, commonly used to control nausea and vomiting, is an oral neurokinin-1 receptor antagonist, which blocks the mast cell degranulation mediated by NKR-1.
Aprepitant, commonly used to control nausea and vomiting, is an oral neurokinin-1 receptor antagonist, which blocks the mast cell degranulation mediated by NKR-1.
Evidence for aprepitant in the treatment of severe pruritus
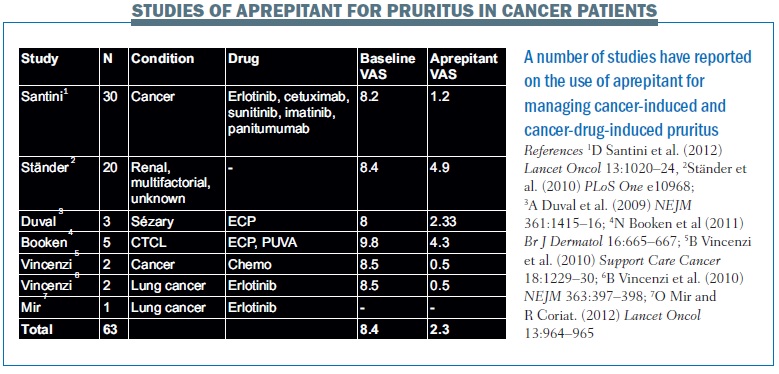
The first report that aprepitant was able to reduce pruritus was published by Duval in 2009 (NEJM 361:1415–16). Three patients with Sézary syndrome had pruritus as the main symptom, with a severity that decreased their quality of life, scoring 7, 8 and 9 on a pruritus visual analogue scale (VAS). The pruritus could not be controlled with conventional therapy. Treatment with aprepitant at a daily dose of 80 mg was associated with a reduction in the VAS scores to 2, 3 and 2, respectively.
A publication from our group reported on two patients – a man with metastatic soft tissue sarcoma and a woman with metastatic breast carcinoma – who were receiving systemic chemotherapy. Both had pruritus that was resistant to local application of corticosteroids and to systemic treatment with antihistamines and corticosteroids. Treatment with aprepitant (standard doses: 125 mg on day 1 and 80 mg on days 2 and 3) was associated with significant improvement of pruritus in both patients 24 hours after the first administration (Support Care Cancer 2010, 18: 1229–30).
Two years ago we published results that demonstrated efficacy with aprepitant for the first time in erlotinib-induced pruritus. Two patients – a woman aged 44 years and a man aged 74 years – with stage IV non-small-cell lung cancer treated with erlotinib (150 mg once daily) had an acneiform rash (grade 3) resistant to steroids, with VAS scores for pruritus of 8 and 9 (NEJM 2010, 363:397–398). Erlotinib was discontinued for one week and then restarted at a lower dose of 100 mg once daily, given three times over one week, on the first, third and fifth day. The patients were also given prednisone and antihistamines, but they relapsed with severe pruritus (grade 3). However, both patients showed prompt recovery from pruritus 24 hours after the first administration of aprepitant.
After two months of treatment with erlotinib with aprepitant prophylaxis, no further episodes of severe pruritus were recorded. VAS scores for pruritus were 0 and 1. This finding was very important for us, because we had not thought that the effects of the aprepitant would last as long as they did.
An overview of clinical publications reporting the use of aprepitant for the management of pruritus induced by cancer and cancer drugs shows a consistent reduction in VAS scores (see table below).
 Prospective pilot study of aprepitant
Prospective pilot study of aprepitant
I think that one of the most interesting publications was from our research group, published in Lancet Oncology in 2012 (vol 13, pp 1020–24). It was a phase II prospective pilot study in 35 cancer patients aged 18 years and over with a histologically confirmed diagnosis of a solid tumour. They had severe pruritus, with a VAS score of 7 or more during treatment with an anti-EGFR antibody or TKI. Two populations received aprepitant: the first was resistant to at least one week of systemic treatment with steroid and/or antihistamine, and the second was naïve, suffering a first occurrence of severe pruritus.
Exclusion criteria were: oral treatment with antimycotics during the four weeks preceding enrolment; topical treatment during the previous two weeks; concomitant chronic renal or hepatic insufficiency, which can both induce pruritus; and concomitant skin infection or dermatitis that would have reduced the possibility to evaluate the effect of aprepitant.
In the group of patients with steroid- and/or antihistamine-resistant pruritus with a VAS score of 7 or more, aprepitant was administered (125 mg on day 1; 80 mg on days 3 and 5) after one week of standard systemic treatment (prednisone 25 mg/day and/or fexofenadine 180 mg/day).
In the second group, the naïve patients, aprepitant was administered (125 mg on day 1, 80 mg on days 3 and 5) directly after the first onset of severe pruritus (VAS score ≥7). We measured the effect of the aprepitant using the VAS score before and after aprepitant was administered, on day 0 and day 7, and then at weekly intervals, until the biological therapy ended or pruritus recurred. The VAS score was registered in a diary given to each patient before starting the study, and noted every week throughout the study period. Response was defined as greater than a 50% reduction of pruritus intensity in comparison to the baseline value.
In terms of patient characteristics, the study included 24 patients in the refractory group and 21 patients in the naïve group. Lung cancer was the most common type of solid tumour, but 33% of the refractory group and 24% of the naïve group had colorectal cancer, and 17% and 19% of the respective groups had other types of cancer. The targeted therapy inducing pruritus was erlotinib in 46% of patients in the refractory group and in 24% of the naïve group; cetuximab was the treatment in 42% of the refractory group and 62% of the naïve group.
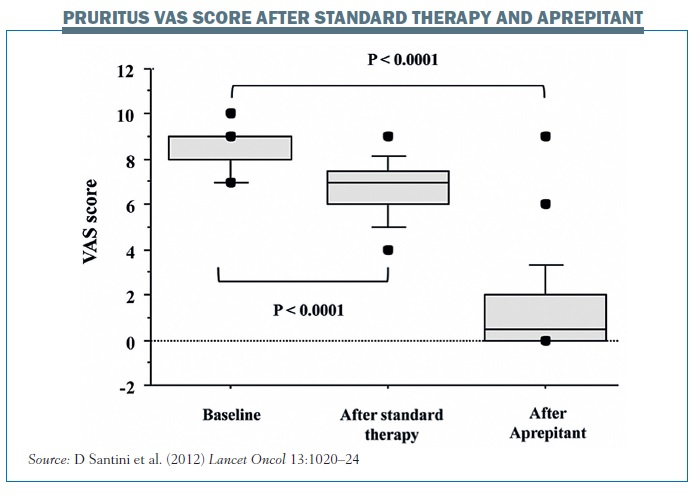
The results showed a statistically significant reduction in pruritus with aprepitant. The 24 patients in the group who were resistant to steroids/antihistamines had a median baseline score for pruritus intensity of 8, indicating a very high intensity of pruritus (95%CI 7.93–8.57; range 7–10; mean 8.25±0.79). After a minimum of one week of systemic therapy the median pruritus intensity decreased by a median of 23% to 7 (95%CI 6.21–7.19). However, after one week of aprepitant therapy the median pruritus intensity decreased from 7 to 1 (95%CI 0–2), representing a 93% reduction (range 0–100%; mean 81.6%), which was highly statistically significant (P<0.0001, Wilcoxon test). The figure opposite summarises the results in patients with pruritus resistant to standard therapy at baseline score, after one week of steroid therapy and then after one week of aprepitant.
We saw two subpopulations of patients with refractory pruritus: the group treated with cetuximab and the group treated with erlotinib. In the cetuximab-treated population (10 patients) the median value of pruritus intensity at baseline was 8, and this decreased by 24% after standard treatment and by 93% after treatment with aprepitant, so its effect was similar to that in the study group overall. In the patients with erlotinib-induced pruritus, we saw similar results, with aprepitant reducing the pruritus VAS score to 1, with a median 85% reduction in the intensity of pruritus. Patients treated with other biological therapies – gefitinib, imatinib and sunitinib – showed similar reductions in pruritus score with aprepitant.
In the naïve group of 21 patients, the intensity of baseline pruritus was also 8 (95%CI 7.43–9.37), so it was severe. After more than one week of aprepitant the median pruritus score decreased by 100% to a median of 0 (95%CI 0.06–1.08; P<0.0001), with an immediate decrease in intensity that was very similar whether the pruritus was induced by cetuximab (n=13) or erlotinib (n=5). Patients treated with other agents showed similar reductions in pruritus with aprepitant.
Another important factor to explore was the time to recurrence of pruritus: how long did the effect of aprepitant last? In our study, we found that only six patients (13%) experienced a recurrence of pruritus, with a median interval of seven weeks from the first administration of aprepitant. These patients had a further treatment cycle with aprepitant (four patients receiving cetuximab, one receiving erlotinib and one lapatinib) and showed a median decrease of 88% in pruritus. None of these patients developed any further recurrences. In our practice we have observed third and fourth recurrences of pruritus and these often, although not always, respond to further administration of aprepitant.
It is important to pay particular attention to the risk of drug–drug pharmacokinetic interactions because aprepitant can alter the activity of cytochrome P450 3A4 isoform (CYP3A4), an enzyme involved in the metabolism of a range of anticancer drugs, including tyrosine kinase inhibitors (Lancet Oncol 2012, 13:964–965).
Conclusions
In conclusion, pruritus is common when we use biological therapies in the treatment of cancers and, when moderate or severe, it can decrease patients’ quality of life. We have demonstrated that aprepitant is effective in reducing severe pruritus induced by biological therapies in cancer patients, both in naïve and refractory pruritus. I consider that this is likely to be a class effect rather than a specific drug effect. The reduction in pruritus with aprepitant is generally long lasting – about seven weeks – in most patients, although some may have recurrences of pruritus. Aprepitant is effective in reducing pruritus irrespective of the cause, showing efficacy in patients with pruritus induced by cetuximab or erlotinib. We have not observed any toxic effect related to potential pharmacokinetic interactions between aprepitant and TKIs metabolised by the same liver cytochrome enzymes in clinical practice.
 Question & Answer
Question & Answer
Fausto Roila, chair of the Medical Oncology Division at the Santa Maria Hospital, Terni, in Italy, hosted a live question and answer session
Q: Your study, like other studies, enrolled only patients with severe pruritus and none with moderate pruritus. Do you think that moderate pruritus – pruritus that is intense, interferes with the patient’s quality of life and requires oral treatment – could be treated in the same way as grade 3 pruritus or not?
A: The problem is that it is not always easy to separate severe from moderate pruritus because it is a subjective symptom. Moderate pruritus is more frequent than severe pruritus, and there are many studies in dermatological settings showing that moderate pruritus reduces quality of life. I think that these patients should be treated with steroids, but moderate pruritus is sometimes refractory to steroids, so it would be interesting to study neuro-kinin inhibitors in moderate pruritus.
Q: Only 45 patients were enrolled in your study on pruritus, so I think these results need to be confirmed in a larger trial. What do you suggest as comparator drugs for aprepitant in both patient populations – those with refractory pruritus and in those untreated?
A: I think we need to do two different studies, one in naïve patients and the other in refractory patients. Naïve patients should be randomised to standard treatment with antihistamines and/or steroids or to aprepitant. Refractory patients should continue antihistamines and steroids and should be randomised between aprepitant and placebo. These two different studies could help us demonstrate that neurokinin inhibition is able to reduce pruritus in cancer patients treated with biological therapies.
Q: Do you suggest preventative treatment in patients treated with EGFR inhibitors or not?
A: I think that is another field of research. There have been no studies demonstrating the use of standard therapy in the prevention of pruritus. When we use antibodies against EGFR, for example cetuximab, panitumumab or erlotinib, moderate to severe pruritus occurs in about 20% of patients, so it could be interesting to investigate the preventive use of aprepitant in patients treated with these drugs.
Q: In the meta-analysis and studies evaluating EGFR inhibitor-induced pruritus, the incidence of pruritus is about 22% of patients globally and severe pruritus occurs in about 2% of patients. On this basis, how is it possible to enrol patients with severe pruritus for the studies you have suggested, because we would need to screen about 2500 patients to enrol 45?
A: The meta-analysis considered all biological therapies, so the median incidence of grade 3/4 pruritus for all biological therapies was 1.4%. In our analysis we used mostly cetuximab and erlotinib, and studies with these two drugs show the incidence of severe pruritus is about 10–15%. Also, some of the patients in our study were considered to have moderate pruritus, because we included everyone with a pruritus VAS score of 7 or more, whereas only scores of 8 or more count as severe.
Q: In your study aprepitant was safe, with no particular adverse events reported, but you outlined the potential for drug interaction because of CYP3A4 metabolism of the EGFR inhibitors, which can be reduced by interaction with aprepitant. Perhaps the next study should include pharmacokinetic evaluation of the plasma levels of EGFR inhibitors before starting aprepitant and after its administration, to investigate and avoid the possible risk of increased toxicity.
A: We started a new randomised phase II trial with another neurokinin inhibitor about two months ago, and we are evaluating the pharmacokinetics to check for any interaction between this neurokinin inhibitor and TKIs. This is very important for TKIs, although not for cetuximab or panitumumab, so this is an important issue to study. However, in our practice we did not observe any increase in toxicity related to TKIs, perhaps because the dose of aprepitant was very low because we used a median of three administrations of aprepitant every month.
Q: Do you think that antihistamines and a course of steroids are effective in controlling radiotherapy-induced pruritus for skin irritation and skin toxicity?
A: I think that topical treatments are the most effective treatments to reduce pruritus induced by radiotherapy. However, it depends if you use radiotherapy with biological therapies, because there are some studies showing that the skin toxicity induced by radiotherapy can be increased by the activity of biological therapies and it could be a synergistic effect.