In expert hands, diagnostic multiparametric MRI is more effective than the dreaded prostate biopsy as the first step in identifying prostate cancers. It is certainly less unpleasant. Guidelines and practice are changing to reflect this, but concerns about capacity, access and risk stratification will need to be addressed, writes Simon Crompton.
When Brian Kavanagh saw a urologist after a blood test revealed an elevated PSA (prostate specific antigen) level, the immediate recommendation was a TRUS biopsy. He returned to hospital to have the procedure a few days later, and the experience still lives with him eight years later. First he had to wait two hours. Then the procedure was excruciating. “It was medieval,” he says.
He was so shaken afterwards that he fainted as he left the hospital. Then a few days later, he was re-admitted with what turned out to be septicaemia, an infection caused by the biopsy. It took him three months to shake it off – three months during which Brian felt faint, weak, shivery and unable to live life normally. “It was almost as if the infection was at the core of your being, and it was really frightening,” he says.
“I was just devastated by the whole experience,” says Kavanagh, who is Chairman of the Irish prostate patients’ organisation, Men Against Cancer. “I can’t say I was devastated for life, but I was devastated during the experience.”
Having a TRUS (transrectal ultrasound guided) biopsy is the standard procedure following a raised PSA reading. It involves inserting an ultrasound probe into the rectum and then, guided by the ultrasound images, firing a fine needle along the probe, through the rectum wall and into the prostate, to remove a tissue core. This happens many times – usually 12 – as the doctor takes samples from different prostate areas.
Kavanagh ended up having a prostatectomy and is now symptom free. But he isn’t alone in finding the experience deeply unpleasant – and potentially life changing.
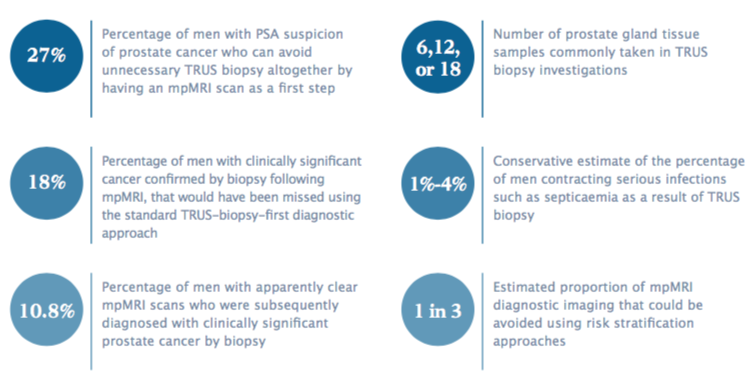
Information given to patients and clinician-authored papers normally stress the rarity of significant side effects, and stress that common complications such as pain, urinary retention and blood in the urine and semen are “typically mild and self-limiting” (see for instance, Eur Urol 2013, 64:876–92). Yet serious infections such as septicaemia occur in 1%–4% of men having biopsies. This is sometimes associated with the development of prostatitis, which around 2% of men experience after biopsy.
Given that these are investigative procedures on largely healthy men, not cancer treatments, such percentages are worrying.
Sadly, studies seldom look beyond the short-term consequences of biopsy. Anecdotally, men have reported that the effects of prostatitis continue long after biopsy, leaving them with long-term pelvic pain and urination problems. A 2017 study indicated that 1 in 20 men regret having a biopsy (BMC Urol 2017, 17:11). Under-reporting is also likely, if Brian Kavanagh’s experience is anything to go by.
A sea change is underway in prostate cancer diagnostic procedures throughout Europe
“I suppose when I got over the septicaemia, I didn’t want to revisit it. It was past, I was better again, and I didn’t want to dwell on it or take it up with the consultant. When you get well you just want to be well.”
Recent research has indicated that all this may be unnecessary, and that biopsy is no longer the best first port if there is a risk of prostate cancer. Major studies have provided compelling evidence that carrying out multiparametric MRI (mpMRI) scans before biopsy is the most effective way of detecting the presence of prostate cancer – making thousands of unpleasant biopsies unnecessary. It also provides highly accurate guidance for biopsy if the scan does identify suspicious lesions.
So compelling is this evidence that mpMRI before biopsy is now becoming the new standard of care for diagnosis in England. Norway and other countries in northern Europe are moving in the same direction. Now the European Association of Urology (EAU), which sets the standard in urological clinical practice in Europe and beyond, is revising its diagnostic guidelines to make similar recommendations. A sea change is underway in prostate cancer diagnostic procedures throughout Europe.
What are the implications?
The impetus for change has come from the PROMIS (Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer) and PRECISION (Prostate evaluation for clinically important disease: sampling using image guidance or not) trials, both run from University College London Hospital.
The multicentre PROMIS trial, involving 740 men with clinical suspicion of prostate cancer and no previous prostate biopsy, tested whether an mpMRI scan before biopsy could identify men who might safely avoid a biopsy. It found that using mpMRI to triage men might allow more than one in four men referred on suspicion of prostate cancer (27%) to avoid a primary biopsy.
If subsequent TRUS biopsies were directed by mpMRI findings, up to 18% more cases of clinically significant cancer (measured by the Gleason score) might be detected compared with the standard pathway of TRUS biopsy for all. A linked study found that an mpMRI-first strategy is effective and cost-effective for diagnosing prostate cancer.
The PRECISION trial went on to look further along an mpMRI-based diagnosis pathway, investigating the accuracy of mpMRI in guiding biopsies, when suspicious lesions have been identified through scanning. The study randomly allocated 500 men with suspected prostate cancer from 23 international centres and found that using mpMRI to perform prostate biopsies led to significantly more of the harmful prostate cancers and significantly fewer harmless cancers being diagnosed, compared to standard TRUS biopsy.
I’m going for a TRUS biopsy ‒ what’s it like?
Accounts of the experience of TRUS biopsy suggest levels of pain and short-term e ects vary widely. These comments, taken from the Prostate Cancer UK Online Community site, were posted in response to the question: “Going for a TRUS biopsy next Tuesday. Anyone share their experiences?”
- “The thought about what’s going to happen was far worse than the actual experience.”
- “The anaesthetic seemed to have no e ect and the surgeon had to stop after the eleventh sample because I was about to have a heart attack, following which I spent a day in hospital. We are all di erent and respond in di erent ways.”
- “When my consultant suggested I had a TRUS biopsy last summer, after doing a bit of research, I was reluctant, not because of the procedure but more the uncertainty of getting an accurate result. More research lead me to the PROMIS trial, which I got my GP to refer me to. I had an MRI, TRUS biopsy and template biopsy. All biopsies were under general anaesthetic so no discomfort, although I ended up being catheterised for a week cos I couldn’t pee!”
- “For me it was a painful experience, but don’t let that put you o . It has to be done and I was probably unlucky on the day or they plain forgot the anaesthetic.”
- “Pretty straightforward, slight discomfort. Be aware that you may have blood in your urine, motions and semen that will take a few days to clear.”
- “I never felt anything. You will be peeing blood for about two weeks afterwards, but it’s only a mild inconvenience.”
Source: Community Prostate Cancer UK
When the PRECISION results were simultaneously announced in the New England Journal of Medicine and at the EAU conference in Copenhagen in March 2018, it felt like a tangible moment of change. “Everyone in the packed eURO auditorium knew they were witness to a practice-changing presentation,” blogged Australian urologist Declan Murphy, “and the swift reaction on social media around the world confirms this.”
The research was the culmination of years of global studies indicating the effectiveness of mpMRI scanning in prostate cancer diagnosis. And it is in the UK where progress is fastest to making it the gold standard. In December 2018, the UK’s health technology assessment body, the National Institute for Health and Care Excellence (NICE), recommended mpMRI as the first-line investigation for people with suspected clinically localised prostate cancer. This follows NHS England publishing a new pathway for diagnosis of prostate cancer in April 2018, revolving around early mpMRI.
“We are seeing rising incidence of prostate cancer, but very little change in the mortality rate,” said Hashim Ahmed of the NHS England Clinical Expert Group for Prostate Cancer. “Our current diagnostic pathway for prostate cancer needs urgent change. The PROMIS trial has shown us that transrectal ultrasound-guided prostate biopsies are inaccurate. They miss significant cancer, overdiagnose insignificant cancers, which leads to overtreatment harms and costs, and biopsies carry risk.”
That same trial, he added, showed that using pre-biopsy mpMRI diagnosed over 90% of significant cancers and fewer insignificant cancers.
The EAU is following close behind. Its 2017 prostate cancer guidelines, compiled with the European Society for Radiotherapy and Oncology (ESTRO), recommended mpMRI for men who had had a previous negative biopsy, but said it was too early to make recommendations on routine use of mpMRI before first biopsy. With the publication of PRECISION and other studies, that situation has now changed, and EAU representatives have said at meetings that mpMRI will be recommended as the new diagnostic gold standard in 2019.
Officially, EAU is keeping its cards close to its chest. In a statement to Cancer World it confirmed that its guideline group has reviewed recent work on mpMRI and anticipated changes to its diagnosis guideline. But there will be implications both for provision of service, and for ensuring appropriate standards and expectations for patient care, said James N’Dow, Chairman of the EAU Guidelines Office. “This means that we need to be sure that the recommendations we issue are robust and evidence-based, and that takes time and care. So we are still in the final consultation phase.” The guideline group is working towards publication at the EAU annual congress in Barcelona in March 2019.
What’s the downside? Some reported long-term effects of biopsy
Literature about the e ects of TRUS biopsy tend to emphasise that side e ects are short-lived and minor. However, studies tend to concentrate on the short term, and the anecdotal experience of many men suggests that lasting physical and psychological e ects may not be uncommon. The comments below were posted on the Harvard Medical School health blog after the editor of Harvard Men’s Health Watch posed the question: What’s the downside to a biopsy?
- “I wish I would have never had biopsies done. I think it has contributed to my ongoing di culty urinating and my lower libido.”
- “At 70, I had my second prostate biopsy. Four months later, I’m still recovering from an infection from that biopsy. Two days after the biopsy I went into the hospital because I had a fever of 103.5 [39.7°C]. They kept me in for ve days, but two days later the fever came back. Back into hospital, this time for 12 days of intravenous antibiotics. Four weeks after being released, the infection came back. I am taking antibiotics at home at the moment. I urge you to avoid a biopsy if at all possible!”
- “I had a prostate biopsy two months ago. The results were negative, but I have been getting more and more ill ever since. I have nausea, weakness and chills since, and it seems to be getting worse.”
- “Four weeks after my biopsy I had a prostate infection. I went back to the doctor for a shot and 10 days’ worth of antibiotics. I never had a fever but I had lingering pain and couldn’t sit on a bike. No cancer was found, but now I think I may be developing erectile dysfunction. I wasn’t prepared for this. The doctor said this will go back to normal, but I’m not sure…”
- “I had a template biopsy six months ago. Now I have erectile dysfunction and prostatitis. My PSA before the biopsy was 3.4. Now it’s 9.1. I seriously regret having it done. I’m aged 54.”
Source: Harvard Health
As EAU appreciates, the implications of a Europe-wide change are huge. Even in England, where uniformity of provision has been imposed by a National Health Service, and multidisciplinary working and systems for mpMRI after first biopsy are already well established, there’s a tacit acknowledgement that reform won’t be easy. Introducing the new prostate cancer diagnostic pathway for England, Hashim Ahmed said it was a “watershed moment”. “I trust all of us will fully embrace the change.”
The rest of Europe will also have to address issues of capacity, professional working relations, training and culture. Some health systems will be better suited than others. Amid widespread acknowledgement that the change is necessary, there are worries: about the scale of the investment and training required, and about the dangers of embracing techniques that are still emerging.
Caroline Moore, Reader in Urology at University College London, and senior author on the PRECISION study, says she is “more than delighted” that the EAU guidelines will be changing. This will certainly put Europe ahead of the USA, she says, where professional guidelines only view mpMRI as useful rather than essential.
But she is also all too aware that mpMRI is a complex procedure – both when used to initially spot lesions, and then to guide biopsies. The main challenge, she says, is one of quality. “It’s very easy to do bad MRI,” she says.
Unlike introducing a new drug, she explains, introducing mpMRI is not automatically standardised. The scanners in each unit will need to be assessed and optimised for prostate cancer. Reporting on the initial mpMRI will need quality standards and guidance – whether done through diagrams, notes or imaging software, it needs to be genuinely useful in guiding biopsies.
Some countries will inevitably find the transition more challenging than others. It’s not simply a question of having the technical resources, capacity and skills – though these will clearly determine the rate of change in many countries. It’s also a matter of professional cultures and working relationships: introducing the new procedures may involve changes in working practice and a reorganisation of professional roles between radiologists and urologists. Some may feel threatened, as TRUS biopsy is no longer at the heart of diagnosis.
The change may be easier in countries with a history of multidisciplinary working, and with greater specialisation in prostate cancer. In Germany, where there has been recent movement towards a national system of specialist prostate cancer units, mpMRI is currently not widely used in diagnosis, but its use is increasing, according to Günter Feick of the German patients’ association Bundesverband Prostatakrebs Selbsthilfe.
“The main challenge is one of quality – It’s very easy to do bad MRI”
German interdisciplinary guidelines for the early diagnosis of prostate cancer currently say that mpMRI can have a role in initial prostate cancer diagnosis, but do not recommend routine use. They point to one of the more worrying PROMIS study findings: 10.8% of men with apparently clear mpMRI scans were subsequently diagnosed by biopsy with clinically significant prostate cancer.
This is also a concern for Riccardo Valdagni, Director of the Prostate Cancer Programme and Chair of the Prostate Cancer Unit at Fondazione IRCC at the National Cancer Institute in Milan, Italy. He says that, in general, it is clear the mpMRI is the way ahead. But he is concerned about biopsy studies showing that 10–20% of men with apparently clear scans actually have small-volume, but aggressive, cancers. Although this is a considerable improvement on TRUS, and although definitions of ‘clinically significant’ vary, this highlights the danger of embracing the technique without also being aware of its predictive limitations, and without conducting further research to overcome them.
Because of this, he believes that – for the time being at least – men with a higher risk of prostate cancer (for example because of family history or a PSA level above a certain threshold) should have a biopsy even after a negative mpMRI scan. The negative predictive value of the technique needs to be moving towards zero, he says – and this means improving technology, specialisation and expertise. Given wide variations throughout Europe, this means the adoption of mpMRI in prostate cancer diagnosis is likely to be very gradual, with expertise slowly spreading from centres of excellence.
“Around 90% of the work on mpMRI in prostate cancer has been done in academic centres,” says Valdagni, “and we know from their studies that the reproducibility of the methods is variable among other academic centres and in real life clinical experience. This is because we don’t really have standardised methods to store and analyse data on mpMRI, and that the learning curve is long.
“You can consider the situation similar to what happened 40 to 50 years ago with breast mammography, starting in one place and little by little moving and expanding across the whole nation and Europe.”
As far as Italy itself is concerned, Valdagni says the past three years have seen a growing demand for mpMRI from GPs, physicians and urologists, accompanied by an explosion in units providing the scans. “Obviously there is still much work to do, but I would say the growth is really satisfying and the technology is present from north to south.”
mpMRI vs TRUS biopsy in numbers
According to Monique Roobol, an epidemiologist and Professor of Decision Making in Urology at Erasmus University Medical Centre, Rotterdam, a European divide is already emerging. Western and northern Europe countries are already implementing diagnostic mpMRI, sometimes on a widespread basis, whereas eastern and southern European countries have far patchier availability, often limited to larger cities. Radiologist Rowland Illing, Chief Medical Officer for a company that markets imaging and cancer detection services across Europe, says that limitations in resources and expertise mean very little is happening at state level across central and eastern Europe to develop diagnostic mpMRI. “When guidelines change to direct patients to MRI before biopsy, it is unlikely to change practice on the ground any time soon,” he says.
In the Netherlands, by contrast, more than half of larger centres are already implementing a pre-biopsy mpMRI policy, says Roobol, and there are very few centres that do not provide access to mpMRI for prostate cancer diagnosis – either directly or by referral.
It’s not simply about having the technical resources, capacity and skills… It’s also about professional cultures and working relationships
The EAU and the European Union will need to address such national disparities, she says. “There’ll need to be not just investment but training programmes – in the same way that there are already, for example, training programmes in robotic surgery. Luckily, there are already courses on interpreting mpMRIs and performing mpMRI TRUS fusion biopsies. Similar activities can be seen at the radiology associations – which is good, since personnel dedicated to interpreting mpMRI images in prostate cancer is a must, just as there are prostate experts in pathology. A lot of the data published represents expert centres and we must be certain that, if we implement mpMRI and targeted biopsy into daily clinical practice, quality is assured.”
At the same time, and mainly because resources will always be an issue, unnecessary mpMRI testing should be minimised. Although it is possible that the EAU will recommend pre-biopsy mpMRI for all men with elevated PSA levels, Roobol advocates risk stratification to determine who is most likely to benefit.
“There’s a lot of unnecessary TRUS testing already, and if we go the same way with mpMRI then I am against it,” she says. National guidelines in the Netherlands provide risk calculators so that unnecessary pre-biopsy mpMRIs are avoided. For example, PSA density readings (not simply PSA levels) and risk factors such as having had a previous negative biopsy and family history are added into a calculation, which provides a prediction of the likelihood of clinically significant prostate cancer.
“We can save at least a third to half of mpMRIs doing this. And if that would happen across Europe, in daily clinical practice, and we really start to stratify risk, then I’m totally for using the mpMRI before biopsy.”
Roobol’s qualified welcome of the new diagnostic world for prostate cancer reflects many other specialists’ ambivalent views. There is an awareness that, whatever EAU recommends in 2019, the hard work of making mpMRI an effective diagnostic intervention is just beginning. Senior figures like Roobol have seen it all before.
“The research clearly shows the potential benefits,” she says. “But we have to keep monitoring. There are constant waves of change in prostate cancer diagnosis. We saw it in PSA cut-offs, which started with PSA above 4.0 ng/ml and ended with 3.0 ng/ml or even 2.0 ng/ml. We saw it in TRUS biopsies, where we started with six cores and now we have 18 or even 24 cores. Then we concluded that this is not going very well because we have overdiagnosis.
“If we really start to stratify risk, then I’m totally for using the mpMRI before biopsy”
“The next wave was active surveillance for increasingly wide groups of men. Things get implemented like crazy, and then we say, ‘Oh my God, perhaps it’s not the improvement we anticipated. Let’s go back again a bit.’ So we must monitor what’s going on with mpMRI, and make sure that we follow up to find out what happens to patients in the long term.”