Brachytherapy, a standard of care for some cancer indications, which involves delivering radiotherapy from a source within the tumour, is underfunded, increasingly marginalised and in decline across much of Europe. Janet Fricker talked to specialists from ten countries, and asked why is this happening, does it matter, and what can be done about it?
In the past few years a spate of editorials have been published in medical journals with emotive headlines including “Brachytherapy: where has it gone?”, “Resurrecting brachytherapy from brink of oblivion” and “Brachytherapy: a dying art or missed opportunity?” Such coverage raises questions about whether brachytherapy is truly moribund, and if so should efforts be made to rekindle its flame?
To gain a snapshot of trends in brachytherapy use across Europe, and explore whether the current patterns of use are optimal in terms of benefit to patients and sustainability of health services, Cancer World spoke to radiation oncologists from nine European countries, and one from the United States – almost the sole source of data on trends in use.
The picture that emerged reveals that use of brachytherapy does appear to be declining in most countries we spoke to; the decline is not primarily driven by evidence of patient benefit or value for money; and many patients who might benefit are never offered brachytherapy as an option.
Many patients who might benefit are never offered brachytherapy as an option
Three common themes emerged: challenges in the education of the next generation of radiation oncologists; the need to rebrand the image of brachytherapy; and the farce of achieving adequate reimbursement for brachytherapy.
Brachytherapy is a form of radiotherapy involving placement of a short-range radioactive source within or close to the site of cancer. Dating back to the discovery of radioactivity in 1898, it represents one of the oldest forms of radiotherapy, but perhaps the least known today.
In the past few years, the technology has undergone a period of renaissance. Modern brachytherapy consists of a series of steps involving insertion of applicators (catheters or needles) to transmit the radioactive material into the patient’s body, followed by delivery of the radioactive source into the applicator by the afterloader device, which stores the source safely between use. Image acquisition, using ultrasound, CT or MRI scans, supports physicists using complex software to plan treatment to sub-millimetre precision, precisely contouring the radiation to the volume needing treatment.
Austria: fewer procedures done in regional centres
“Around 12 of Austria’s 14 radiotherapy centres perform brachytherapy, with the highest number of procedures done at the Medical University of Vienna. Top indications for brachytherapy are prostate, cervical/endometrial, breast, anal, rectal, head and neck, skin and bronchus, in that order. The Medical University of Vienna has a strong tradition of brachytherapy research and coordinates EMBRACE, a multicentre study of MRI-guided brachytherapy in locally advanced cervical cancer.
“In Vienna, the number of brachytherapy patients we treat has remained more or less stable, although this is probably due to increased referrals from regional centres, where numbers are consequently falling.
“We feel it is important for patients to be treated by brachytherapy specialists with experience performing a high number of similar procedures each year. Just because they perform brachytherapy for one indication doesn’t mean that they know how to perform it for another. Such levels of specialisation can create training issues, however.
“Radiation oncologists trained at radiation oncology institutes may not have been exposed to all types of brachytherapy. We need to find a way to achieve a balance.
“In Vienna, we’ve no problems with brachytherapy reimbursement, which is calculated according to the number of publications and trainees. However, we believe that regional reimbursement levels fail to take into account the true costs of brachytherapy to the service.”
Christian Kirisits, Medical University of Vienna
According to Bradley Pieters, a radiation oncologist from the Amsterdam Academic Medical Centre, who chairs the GEC–ESTRO Brachytherapy Committee, the great advantage of brachytherapy lies in its “unparalleled ability” to direct large doses of radiation to the tumour while more or less sparing healthy tissue in the neighbourhood. Placing the radiation source inside the affected organ also has other benefits. It avoids the hazards of aiming for a moving target, which can skew dose delivery with external beam radiation if the tumour shifts position with the movement of a patient’s breathing, or normal changes in their bowels or bladder. It also avoids the prolonged period of frequent hospital visits usually required for courses of external beam radiotherapy, thereby enabling patients to get on with their lives, and reducing adherence problems.
Downsides include the need for local or general anaesthesia, the risks of bleeding and infection involved in any invasive procedure, and challenges posed by access to the tumour site.
The most common use of brachytherapy is for cervical and prostate cancers, but it can also be employed for breast, bladder, oesophageal, head and neck (lip, tongue, cheek and tonsil), lung, gallbladder and anal cancers.
Czech Republic: access depends on who the patient sees first
“Out of 25 radiotherapy centres in the Czech Republic, 15 offer brachytherapy. Studies suggest that around one-third of Czech cancer patients receive radiotherapy, which is considerably fewer than our 50% target, with 7‒9% of these receiving brachytherapy.
“The main indications are for gynaecological cancers, especially cervical and endometrial, which account for 70% of all brachytherapy procedures. Additionally, three to four centres perform prostate and a few head and neck and breast boost, including partial breast irradiation. We also perform limited procedures for bronchial, skin, and penis cancers and soft tissue sarcomas.
“Learning brachytherapy techniques is an integral part of radiation oncology training, with all trainees required to spend at least three months in brachytherapy departments. Stays, however, are limited, making it hard for them to acquire sufficient skills, especially manual skills, to practise independently.
“We would like to rationalise the service, offering it in fewer centres to allow clinicians to acquire greater expertise. However, this is unpopular politically as patients want to have the service located close to their homes.
“Access to brachytherapy procedures is often determined by the clinician who patients consult first, with many urologists and dermatologists being reluctant to refer patients for brachytherapy. Insufficient reimbursement of brachytherapy procedures in particular, as well as radiotherapy in general, is another issue we are facing.”
Hana Stankusova , University Hospital Motol, Prague
Trends in use
The period between the 1930s and 1970s can be considered the golden age of brachytherapy, when invasive radiation techniques represented the main mode of radiotherapy. But advances in external beam radiotherapy, such as intensity modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT), captivated clinical imaginations, resulting in declining interest in the older technology.
Published evidence regarding how this has impacted on brachytherapy use is sketchy, with much of the data coming from the United States. A study of men treated with either brachytherapy or external beam radiotherapy for low-risk prostate cancer, using the US National Cancer Database, showed the proportion treated with brachytherapy declined from 62.9% in 2004 to 51.3% in 2012 (J Contemp Brachytherapy 2016, 8:289–93).
A similar picture has emerged for treatment for locally advanced cervical cancer, with analysis of the Surveillance, Epidemiology, and End Results (SEER) database showing a decline in rates of brachytherapy use following treatment with external beam radiotherapy, falling from 83% in 1988 to 58% in 2009 (Int J Radiat Oncol Biol Phys 2013, 87:111–19). The same study also found that brachytherapy treatment was independently associated with better cancer-specific survival (HR=64, 95%CI 0.57–0.71) and better overall survival (HR=0.66, 95%CI 0.60–0.74).
These findings are a cause for concern says Peter Orio, from the Dana-Farber Brigham and Women’s Cancer Center, in Boston, and current President of the American Brachytherapy Society: “Brachytherapy is an extremely important and valuable tool in the armamentarium to cure cancer. As a profession we’d be taking a huge step backward if we allow something that works so well to disappear simply because it isn’t perceived as being as exciting or sexy as some of the emerging technologies.”
In Europe, the only available data on brachytherapy come from a 2013 review published in Lancet Oncology, which revealed there were 657 brachytherapy facilities, representing 52% of all radiotherapy centres (Lancet Oncol 2013; 14:e79-e86). No European data are available to indicate whether the number of centres offering brachytherapy has fallen or what has happened with throughput of patients.
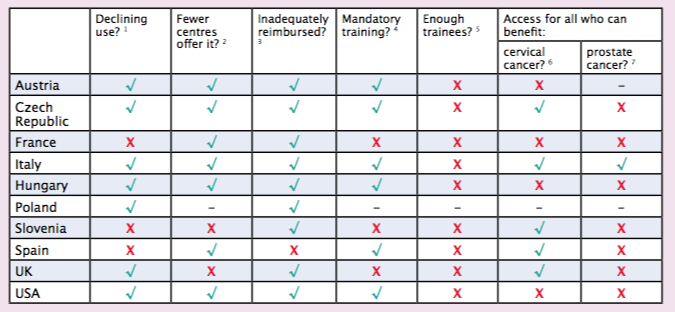
On the basis of responses to questions Cancer World put to radiation oncologists across nine European countries, we can reveal that brachytherapy use is seen to be in decline in Austria, Czech Republic, Italy, Hungary, Poland and the UK, while in Spain, Slovenia and France, levels of use seem to be more stable.
France: many radiation oncologists learn no brachytherapy during training
“Data from the National Cancer Survey show that, out of 182 radiation therapy departments in France, 54 perform some sort of brachytherapy. Forty-one centres offer cervical procedures, 23 prostate and three breast, with brachytherapy also occasionally performed in head and neck, penile, anal and skin cancers and soft tissue sarcoma. In high- and intermediate-risk prostate cancer, we are particularly concerned that only 10 centres offer external beam with brachytherapy boost, which has been shown to deliver better outcomes than external beam alone.
“France used to be famous for brachytherapy. But in 2014, when low-dose sources were removed from the market, many centres were not prepared to buy the high-dose equipment, and stopped offering procedures. This is not necessarily a bad thing, as we feel it would be best to have no more than 30 or 40 dedicated brachytherapy centres in order for radiation oncologists to achieve adequate experience.
“Many radiation oncologists in France have not experienced brachytherapy procedures during training. To address this, the brachytherapy group of the French Society of Radiation Oncology organises annual training programmes to teach 10‒15 radiation oncologists who either missed out on brachytherapy during initial training or want to update their technique. Training includes a theo-
retical component and a practical component, with the chance to practise procedures on a simulator.
“Reimbursement is an issue in France, as no account is taken of the cost difference between easy procedures and more complex procedures requiring imaging and anaesthesia. Some institutions who believe in brachytherapy redistribute money from other sources of funding, but others are not willing to do this, leading to a loss of the service.”
Jean Michel Hannoun-Levi, Antoine Lacassagne Cancer Centre, Nice
We also found that the types of cancers treated by brachytherapy vary markedly across Europe. While most brachytherapy centres offer cervical, uterine and prostate procedures, use in breast cancer is less predictable, with countries such as the UK and Slovenia providing no service for this indication. Whether treatments for indications such as skin, head and neck, penile, anal and oesophageal cancers are offered seems largely dependent on local expertise developed at individual centres. Indications for brachytherapy appear in flux, for example in Spain, where overall brachytherapy use remains stable, procedures for prostate and skin indications have increased, but those for head and neck and gynaecological indications have fallen.
Does it matter?
For certain indications in cervical and prostate cancer, studies have demonstrated that brachytherapy makes a significant difference to overall survival and disease-free survival respectively. In these cases, access to brachytherapy clearly does matter.
Locally advanced cervical cancer
Two retrospective US studies on outcomes in cases of locally advanced cervical cancer, using data from the SEER database (7,359 patients) and National Cancer Database (7,654 patients) respectively, showed that brachytherapy boost versus no boost significantly increased overall survival after external beam radiotherapy, and that using IMRT or SBRT as alternatives to brachytherapy boost also resulted in significantly worse overall survival (Int J Radiat Oncol Biol Phys 2013, 87: 111–19; Int J Radiat Oncol Biol Phys 2014, 90:1083–9).
Hungary: prostate cancer patients are missing opportunity for boost
“In Hungary we have 13 radiotherapy centres serving 10 million inhabitants, with two in Budapest and the rest in the regions. For each radiotherapy centre it’s mandatory to have a minimum of two linear accelerators and one high-dose-rate afterloader, which ensures all centres have the equipment to perform brachytherapy. However, in reality 90% of the centres offer brachytherapy only for gynaecological procedures; other indications such as prostate, head and neck, and breast, are only performed at the national centre. Unfortunately, there’s no requirement for regional centres to refer patients to the national centre, so they often get external beam only. We’re particularly concerned that many prostate cancer patients are missing out on the opportunity for brachytherapy boost, which in some indications is more effective than external beam alone.
“There are two reasons why regional centres may be reluctant to perform brachytherapy. First, radiation oncologists also administer chemotherapy, so they have limited opportunities to get to grips with complex brachytherapy procedures. Second, centres are well aware that brachytherapy treatments are not adequately reimbursed, and they are reluctant to lose money.
“We’ve been compiling a dossier to support proper reimbursement of brachytherapy, including clinical evidence, the true cost of all the different types of treatment, and cost‒benefit ratio comparisons with alternative treatments. This took around two years, and the dossier is now with the national health insurance company. We’re confident that we will be successful as we previously undertook a similar process with CyberKnife that resulted in an increase in reimbursement for stereotactic ablative body radiotherapy. Once brachytherapy is adequately reimbursed, we hope that regional centres will be more willing to offer a wider range of brachytherapy techniques.”
Csaba Polgár, Director General of the National Institute of Oncology, Budapest
Commenting on those and other studies in an editorial titled: ‘Curative radiation therapy for locally advanced cervical cancer: brachytherapy is NOT optional,’ radiation oncologists from the US and Europe raised concerns about evidence indicating that physicians in the United States may be attempting to replace brachytherapy with external beam boosts, arguing that this could lead to unnecessary recurrences, toxicities, and even deaths. “A new drug yielding a 10% survival improvement would be heralded as a great advance. Ironically, it is likely that we could achieve similar improvements in the outcome of patients with cervical cancer by simply applying tried and true radiation therapy techniques using best practice guidelines,” wrote the authors (Int J Radiat Oncol Biol Phys 2014, 88, 537–39).
Prostate cancer
The evidence for brachytherapy in prostate cancer is also well established, used as a monotherapy for low- and intermediate-risk patients and as a boost, following external beam radiotherapy, for higher-risk disease.
Two recent randomised controlled trials have demonstrated significant improvement in biochemical disease-free survival when brachytherapy was used as a boost strategy for patients with higher-risk disease. In patients with high-risk localised prostate cancer, rates of relapse free survival were significantly higher in patients treated with brachytherapy versus no such boost following external beam radiotherapy (Radiother Oncol 2012, 103:217–22).
In patients with intermediate- and high-risk prostate cancer, randomised to a standard arm receiving 12 months of androgen deprivation therapy and pelvic irradiation, followed by dose-escalated external beam therapy, or to an experimental arm substituting brachytherapy for the external beam therapy, biochemical failure was twice as high in the external beam arm at a median follow-up of 6.5 years (Int J Radiat Oncol 2017, 98:275–85). An overall survival advantage has yet to be reported for either study, but, as Orio comments, “The importance of biochemical control cannot be underestimated in prostate cancer treatments, as it triggers a cascade of events that reduce quality of life.”
“We’re concerned that if brachytherapy isn’t made available to some cervical and prostate cancer patients this could jeopardise their chance of achieving good outcomes,” says Pieters. His fears are echoed by the radiation oncologists we interviewed, who reported that in Austria, France, Hungary and the US, for instance, there are women who would benefit from cervical brachytherapy who are not being offered it, while in the Czech Republic, France, Hungary, Slovenia, Spain, the UK and US there are men who would benefit from prostate brachytherapy who are not being offered it.
Italy: many centres with facilities do not provide the service
“The brachytherapy study group of the Italian Association of Radiation Oncology (AIRO) recently undertook a survey to provide a snapshot of brachytherapy services across Italy, to define policy goals (J Contemp Brachytherapy 2018, 10:254‒59).
“One third of radiation oncology centres responded ‒ 66 out of 197. Almost half of the responding centres that are equipped with brachytherapy facilities either do not deliver the service at all, or delivered less than the demand for it, because of lack of staff, or expertise, or up-to-date equipment. The majority of treatments were administered to outpatients for gynaecological indications. Fewer centres provided brachytherapy for prostate, breast or head and neck cancers.
“While we don’t have data to show a decline in brachytherapy procedures, we have a strong sense this is happening. One reason is skill shortages, as radiation oncologists are not given sufficient time in training to gain interventional brachytherapy skills, which take several years to acquire.
“The legal minimum requirements for accreditation of Radiation Oncology schools include brachytherapy practical teaching. However, our own education survey ‒ yet to be published ‒ shows most departments don’t have experts capable of teaching all the potential brachytherapy applications, especially for indications beyond cervical and endometrial cancers.
But we have rediscovered a passion for brachytherapy, in Italy, and are making efforts in education, national clinical guidelines and patient/physician communication. We would like to see specific training courses set up, and a Masters’ qualification in brachytherapy for radiation oncologists.
“The AIRO study group is also involved in managing national guidelines for brachytherapy in specific clinical practice situations, and is developing a national network for research. We believe brachytherapy for frequent applications, such as cervical, endometrial, prostate and skin cancer, should be available in all centres, but rarer indications, such as sarcoma and eye, should be concentrated in a specialist centres, to achieve sufficient volumes to establish expertise.
“Some types of brachytherapy procedures are not adequately reimbursed in Italy.”
Luca Tagliaferri, Fondazione Policlinico Gemelli IRCCS, GEMELLI-ART, Rome
Other indications
Evidence for differences in overall survival favouring brachytherapy for other indications are less clear. “The sophistication of external beam radiotherapy has greatly increased, becoming much more conformal, with the result that there are fewer situations favouring brachytherapy than a few years back,” says Csaba Polgár, Director General of the Hungarian National Institute of Oncology, in Budapest.
In the UK, Li Tee Tan, from Addenbrooke’s NHS Trust, Cambridge, takes a pragmatic approach. “We need to fight the battles that really matter in brachytherapy, making sure it’s available for indications where there are proven advantages over other modalities. It isn’t practical to say everyone should get brachytherapy, because that just won’t happen. The reality is that in some circumstances external beam offers competitive results that can provide sufficiently high doses to small areas,” she says.
For indications beyond cervical, endometrial, and prostate cancer, studies comparing outcomes for brachytherapy versus other radiation modalities are largely lacking. “The problem is that device companies are reluctant to invest in studies around technology that has a limited turnover,” explains Christian Kirisits, a medical physicist from the Medical University of Vienna, who is a past chair of the GEC–ESTRO Committee.
Yet even where evidence of a survival advantage is lacking, the option of brachytherapy as an alternative to daily trips to a radiotherapy centre still offers advantages for many patients, which could translate into a survival benefit if patients forgo the external beam treatment rather than making those journeys.
Poland: attracting young specialists, and access to imaging, are a challenge
“In Poland 36 out of 45 radiation oncology departments offer brachytherapy. We treat almost all types of cancer with brachytherapy according to the GEC-ESTRO and American Brachytherapy Society recommendations. The procedures offered vary according to expertise at individual centres. Almost every centre offers gynaecological tumour procedures; treatment of other cancer types, such as ocular melanoma, is offered in only a few centres. Due to technical difficulties we don’t offer brachytherapy for brain or bladder cancer. Most procedures are done on an out-patient basis by radiation oncologists who must have a specialist degree.
“Although we have no hard evidence from surveys, our general impression is that the use of brachytherapy is declining in Poland. We think this may be due to a tendency to replace it with newer techniques, such as CyberKnife, as well as issues around access to imaging and problems with attracting young specialists. There are definitely patients in Poland who would benefit from brachytherapy but are not getting access.
“The Polish health reimbursement system does little to encourage use of brachytherapy, as the reimbursement does not take into account the location of the cancer or complexity of the treatment. Recently we were forced to stop offering accelerated partial breast irradiation, the SAVI applicator and permanent prostate brachytherapy with seeds, because levels of reimbursement made the service unsustainable.”
Janusz Skowronek – Greater Poland Cancer Centre, Poznań
Professor Skowronek (1964‒2018) sadly died shortly after this interview
Shortfalls in education and training
Many of the radiation oncologists we spoke to are concerned that the decline in numbers of radiation oncology graduates with sufficient training in brachytherapy is feeding into a cycle of fewer doctors feeling comfortable to perform brachytherapy, which in turn means that they are not transmitting their enthusiasm to the next generation.
The country representatives we talked to (Austria, Czech Republic, France, Italy, Hungary, Slovenia, Spain, UK and US) felt that too few young brachytherapists are being trained to support a future service.
Across Europe there is no overall official training programme for brachytherapy. While in Austria, Czech Republic, Italy, Hungary, Spain and the US it is obligatory for radiation oncologists to get some experience of brachytherapy in their training, in other countries, such as France, Slovenia, and the UK, no such training is mandated. Pieters wants this to change. “Not every radiation oncologist needs to be able to perform brachytherapy, but they all need to have covered it in specialist training so it’s firmly on the menu when talking to patients about options.” Not all radiation oncologists, he adds, prove suited to performing the technique. “They need to be surgically minded with good hand–eye coordination.”
“Many urologists and dermatologists know little about brachytherapy, and aren’t referring patients with skin and prostate cancer to us”
When radiation oncologists decide that they would like to perform brachytherapy they require sufficient exposure to make them proficient. “Brachytherapy is an art, it’s a unique type of radiotherapy combining scientific knowledge, advanced manual skills and judgement. Trainees need at least a year to become proficient to practise,” says Pieters. Countries like France and the US, are now coordinating catch-up educational sessions for radiation oncologists who have either missed out on brachytherapy experience in their initial training or need to update their skills.
Slovenia: urologists aren’t telling men about all their options
“Slovenia, a small country with a population of two million people, has one comprehensive cancer centre employing five radiation oncologists who can perform brachytherapy.
“We treat approximately 500 patients with brachytherapy each year, a figure that has remained more or less stable over the past five years. Cervical and prostate are our most frequent sites, with a few cases of skin, anal and eye cancers performed each year. We know that, compared to western European colleagues, we treat fewer brachytherapy locations, but we hope to start offering accelerated partial breast irradiation after surgery in the near future.
“We enjoy good relations with gynaecology colleagues, who refer all relevant cervical cancer cases to us. One of our biggest concerns, however, is that urologists who diagnose prostate cancer aren’t telling men about all their options, and that we only see a small proportion of men we could help.
“Radiation oncology trainees only learn about brachytherapy when assigned to our team; there’s no formal training in Slovenia.
“We’re currently lobbying the National Health Insurance Institute to improve reimbursement for brachytherapy. To this end, we’ve undertaken extensive research, costing out each individual brachytherapy procedure. We are aware that for gynaecological cancers, for example, we’re only getting one third of our actual costs reimbursed.”
Barbara Šegedin, Institute of Oncology, Ljubljana
Educational activities also need to target medical students to make them aware of the technology in their future careers. “Access to brachytherapy is often determined by the clinician patients consult first. We’ve found that many urologists and dermatologists know little about brachytherapy, and as a consequence aren’t referring patients with skin and prostate cancer to us,” says Hana Stankusova, from the department of Oncology and Radiotherapy, at the University Hospital Motol, in Prague.
Profile and image issues
Many radiation oncologists we spoke to raised the ‘image problem’. External beam radiotherapy is currently considered the zeitgeist, while brachytherapy is seen as a much more traditional approach to practising medicine, which may not appeal to younger iPad-orientated generations. “With external beam different members of the team work sequentially, with both the clinicians contouring the target and the physicists planning the dose able to work remotely from home without ever having to see the patient,” says Kirisits. By contrast, he adds, brachytherapy is often seen as an old fashioned approach, with the different members of the team needing to sit together to plan the procedure, and interact as a group in the operating theatre.
The real irony, says Orio, is that the new technologies causing such a flurry of excitement have all been designed to mimic what has been safely done with brachytherapy for many years. “Just because these technologies are shiny and new, regardless of the associated costs, everyone is gravitating towards them,” he says. People forget that brachytherapy has also undergone technical advances. “In the modern world it’s a completely different beast that involves complex imaging and computing,” says Pieters.
“In the modern world brachytherapy is a completely different beast that involves complex imaging and computing”
A number of interviewees argued in favour of changing the term ‘brachytherapy’ to ‘interventional radiotherapy’, which they feel not only sounds more contemporary but would more effectively convey the nature of the technology and help attract young clinicians to train in the technique. The Italian Association of Radiation Oncology has already added ‘interventional radiology’ into the official name of their brachytherapy study group.
Spain: active group has boosted image, new recruits and reimbursement
“Around half of Spain’s 120 radiation oncology departments offer brachytherapy.
“For prostate and skin tumours, brachytherapy is on the rise; while for head and neck and cervix it is declining. We are also starting to use brachytherapy to perform partial breast irradiation for low-risk tumours instead of external beam. It has advantages here, including being quicker, offering better cosmetic results, and above all less irradiation of the heart and lungs.
“Spain’s strong tradition of brachytherapy can be largely attributed to the enthusiasm of the Spanish Brachytherapy Group, which started in 2001, and with 200 members now represents the largest subgroup of the Spanish Society of Radiation Oncology. Each year the group holds a consensus meeting around specific topics in brachytherapy, exploring how different centres perform the technique, and then reaches a consensus about the best approach. Other achievements for the group include writing two brachytherapy textbooks (published in 2008 and online in 2016), which are used throughout the Spanish-speaking world.
“With such a strong community, Spain has no difficulty attracting young radiation oncologists to brachytherapy. It is mandatory for radiation oncologists in Spain to spend at least two months of their four-year training practising brachytherapy, with the result that everyone has had exposure to the technique. Although new techniques of external radiation are attractive for young specialists, they cannot achieve as high local doses as brachytherapy.
“In Spain we don’t have any issues regarding brachytherapy reimbursement, as five or six different levels have been defined that take into consideration the complexity of different procedures.”
José Luis Guinot, Fundación Instituto Valenciano de Oncología, Valencia
Many also argued that conferences and radiation oncology meetings often reinforce the marginalisation of brachytherapy, by side-lining presentations into specialist tracks. Kirisits would like to see this technique better represented in main sessions. “We need a new model where presentations of trial results are scheduled for the main programme to inform everyone about the latest developments, with only the more detailed practical information reserved for specialist tracks,” he says.
UK: we need to centralise services for less common indications
“In the UK most radiotherapy centres offer brachytherapy in some form or other. The most common tumour sites treated with brachytherapy are cervix, endometrium and prostate, which have remained more or less stable over the past few years. Use of brachytherapy for other sites such as anus, oesophagus, lung, and head and neck is in decline.
“With the exception of anal cancers, where brachytherapy may allow preservation of the rectum and avoid a permanent stoma for patients, I’m not unduly concerned by the reduced range of tumour sites as external beam probably achieves very similar results.
“The Royal College of Radiologists has published guidelines stating that medical oncologists should treat a minimum of 10 patients each year with brachytherapy for each tumour site. However, with the services spread so thin, this in practice is often not achievable. In a small country like the UK, where patients don’t have to travel large distances, it may be sensible to rationalise brachytherapy services, concentrating expertise in a few centres, particularly for less common tumour sites.
“In the UK, clinical oncologists undergo a five-year training programme that covers external beam radiotherapy and chemotherapy for all types of tumours. There is often not sufficient time for exposure to brachytherapy as well. Reimbursement is also challenging, with reimbursement for prostate brachytherapy not even covering the costs of radioactive seeds.”
Li Tee Tan, Addenbrooke’s NHS Trust, Cambridge
The reimbursement paradox
While issues regarding training, profile and image can all help explain the decline in the use of brachytherapy across much of Europe, a bigger problem may be that reimbursement levels are typically so poor that hospitals offering the service frequently incur a net financial loss.
Radiation oncologists in Austria, Czech Republic, France, Italy, Hungary, Poland, Slovenia, UK, and US all describe problems receiving adequate reimbursement for brachytherapy from their countries’ departments of health. Of all the countries we spoke to, only Spain reported having no issues with funding, describing a system where a range of tariffs had been devised that take into consideration the complexity of different brachytherapy treatments.
At the heart of the problem, says Polgár, from Hungary, is that “health departments reimbursing treatment have no real understanding of the complexity of brachytherapy and how costs go beyond radiation equipment.” On top of costs of catheters, needles, and radiation sources, you have to factor in the cost of different imaging modalities and the involvement of multiple different health professionals – not just radiation oncologists, but physicists, anaesthetists and nurses.
USA: we could lose brachytherapy services if we don’t take action
“In the US we train around 180 radiation oncologists each year, of whom only 30 go on to use brachytherapy routinely in their practice. Prostate cancer represents the most common technique, followed by cervix and breast, with less commonly performed techniques including head and neck, lung, gastrointestinal and central nervous system.
“A number of US studies have suggested brachytherapy utilisation is on the decline, especially in prostate and cervical cancers. We are particularly concerned by the cervical cancer situation, as studies show there is a 10% decrease in survival when brachytherapy is omitted from treatment. For other cancers, brachytherapy represents a convenient option – patients require fewer treatments than external beam radiotherapy, but it does not make a difference to survival. The difference in number of treatments can make a difference to quality of life for patients who have to travel vast distances for treatment.
“The US fee-for-service payment system, based around the notion that every time a clinician performs a service they can bill for it, undoubtedly represents one of the reasons for the decline in utilisation of brachytherapy. Taking the example of prostate cancer, external beam radiotherapy requires 44 separate treatments over a nine-week period, while brachytherapy only requires one or two treatments. Such big financial differences provide a disincentive to offer brachytherapy. There is hope that the Department of Health and Human Services’ Cost Containment System, scheduled to be introduced in 2020, will improve the situation. The new system, which focuses on quality and treatment outcomes, will penalise clinicians financially if their initial treatment does not work.
“The American Brachytherapy Society is deeply concerned that if we don’t take action we could lose this beautiful delivery method that’s been the cornerstone of radiation for over 100 years. To this end, we’ve launched the 300 in 10 initiative, which aims to train 300 brachytherapists over the next 10 years to prevent declining skills. We’ve also introduced a scholarship programme to fund radiation oncologists and their physicists together to attend residential courses updating them on brachytherapy techniques with the opportunity to practise on simulators. Additionally, with our ‘Know Your Options’ campaign, we are working to educate patients and to empower them to seek all the information they can about treating their specific disease, including radiation therapies and brachytherapy, so that they can make the most informed decisions regarding the care they elect to receive.”
Peter Orio, Chairman of the American Brachytherapy Society, and Dana-Farber Brigham and Women’s Cancer Center, Harvard Medical School, Boston, Massachusetts
Complicating the picture is a wide variation in costs associated with treating different indications, says Tan, from the UK. While the equipment and work flow involved in external beam radiotherapy is largely standardised, the workflow in brachytherapy is far less predictable, making it much harder to estimate costs, she says. “To really drill down on costs you need to collect a lot of additional information such as whether the patient needed to stay overnight or required imaging.”
Reimbursement levels are typically so poor that hospitals offering brachytherapy frequently incur a net financial loss
Jean-Michel Hannou Levi, from the Antoine Lacassagne Cancer Centre, Nice, agrees. “Funders often have no real appreciation that vaginal brachytherapy is an easy technique that just involves placing the source in the vagina, while cervical or prostate brachytherapy are much more invasive, requiring a general anaesthetic,” he explains.
The paradox is that brachytherapy equipment is by far the least expensive of all radiation therapy modalities. Installing a brachytherapy unit costs in the order of €400,000–600,000, compared to €2–3 million for an IMRT linear accelerator and €100–300 million for a proton centre, says Pieters. Such economical equipment outlays feed into the overall value of brachytherapy, he argues, even taking into consideration the additional staffing and paraphernalia needed.
A 2011 US study by Chirag Shah investigating the cost of different treatment modalities showed costs for individual patients with low- or intermediate-risk prostate cancer were $2,395 for low-dose-rate brachytherapy, and $5,467 for high-dose-rate brachytherapy, compared to $23,665 for IMRT (Brachytherapy 2012, 11:441–5).
“We believe inadequate reimbursement is one of the most important factors suppressing use of brachytherapy in Europe,” says Pieters, adding that such penny-pinching makes no sense given the excellent value for money it offers.
Luca Tagliaferri, from the Advanced Radiotherapy Unit of the Gemelli University Hospital in Rome, says that arguments in favour of rebranding the procedure as ‘interventional radiotherapy’ could be relevant here. “The name ‘interventional radiotherapy’… allows health departments to understand instantly that it’s an interventional procedure requiring higher reimbursement.”
The GEC–ESTRO brachytherapy committee is planning to send out questionnaires to all European centres practising brachytherapy to achieve greater clarity about discrepancies between true costs and reimbursement. The questionnaires will first ask about the range of cancers treated with brachytherapy by the different centres, and then focus on different components involved in the way they deliver cervical cancer brachytherapy.
“We had to start somewhere so we chose cervical cancer first,” says Tan, who is leading the questionnaire project. “We’re trying to understand variations in practice for the same indication. Things like the different treatment protocols involved, the number of separate treatments, the amount of time taken up by each staff member, type of imaging used, and whether an inpatient bed was needed.”
Such data can ultimately be used to calculate the health-economic information, such as quality-adjusted life years (QALYs), needed to make a convincing financial case for adequate reimbursement. The questionnaires will reveal the range of real world approaches that are being used to deliver brachytherapy, and which represents the best value. “In order to inform future services in brachytherapy we want to start with the facts and we think our survey represents the first steps to do this,” says Pieters.
Challenges in brachytherapy service delivery
- Are you under the impression brachytherapy procedures are falling?
- Is there a tendency for brachytherapy procedures to be referred to larger centres?
- Do you have problems getting adequate reimbursement for brachytherapy procedures?
- Is it mandatory to have at least some experience of brachytherapy procedures in radiation oncology training in your country?
- Are sufficient young brachytherapists being trained to support the future service in your country?
- Do all patients who would benefit from cervical brachytherapy get the opportunity for treatment?
- Do all patients who would benefit from prostate brachytherapy get the opportunity for treatment?