Massimo Conio looks at the evidence for two alternatives to oesophagectomies–endoscopic mucosal resection and radiofrequency ablation–and discusses which patients should be eligible and where the treatments should be carried out.
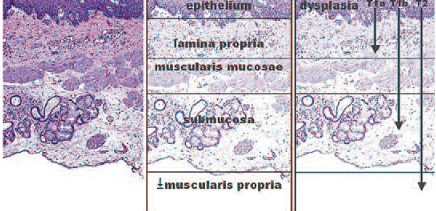
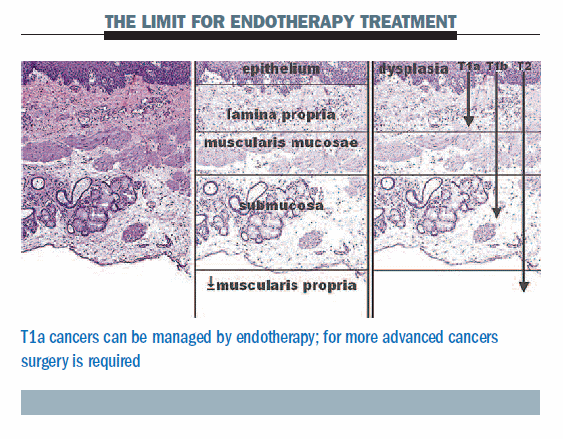
We can safely and effectively use an endotherapy approach in patients who have Barrett’s oesophagus lesions infiltrating to the muscularis mucosae, which means a T1a cancer. The limit of endotherapy treatment is shown clearly in the figure below. Surgery is required for T1b cancers, which involve submucosal invasion of the oesophagus. The most well-known approach for endotherapy is endoscopic mucosal resection (EMR), which enables removal of any malignancy in the mucosa. It also provides an adequate assessment of the histology and enables accurate staging. This approach there-fore enables us to decide what to offer patients as radical treatment.
 A recent study showed how endoscopic mucosal resection can improve staging, with downstaging of the lesion in 28% of cases and upstaging in 20% (Am J Gastroenterol 2010; 105:1276–83). This demonstrates how useful this technique can be.
A recent study showed how endoscopic mucosal resection can improve staging, with downstaging of the lesion in 28% of cases and upstaging in 20% (Am J Gastroenterol 2010; 105:1276–83). This demonstrates how useful this technique can be.
Retrospective studies comparing endoscopic treatment and surgical treatment of muscosal T1a lesions in Barrett’s patients show comparable overall survival (Gastroenterology 2009, 137:815–823). This means that it is no more effective to send a patient with such a lesion to the surgeon to have their oesophagus removed than to treat with endoscopic resection. The major ad-verse effect on quality of life means it is always better to avoid surgery where appropriate.
In the past, resection was used to treat only visible, focal lesions. However, the risk with leaving behind a wide surface of neoplasia is that metachronous lesions might be present. At the beginning, endoscopic mucosal resection was considered mainly for patients of advanced age and those with comorbidities. Until a few years ago the gold standard treatment was still, unfortunately, oesophagectomy, which had the advantage of providing not only the entire specimen, but also of removing all lymph nodes. Things are changing today. In patients with segments shorter than 4 cm, it is now possible to consider removal with mucosectomy or similar techniques.
How is resection performed?
Resection is usually performed by using a plastic cap placed on the tip of an endoscope or using a multiband ligator (MBM). A very simple study showed that the only significant difference between the two approaches was procedure time, which was shorter in the MBM group (Gastro-intest Endosc 2011, 74:35–43). This technique is also considerably easier than oesophagectomy and can therefore be performed in centres with a lower annual number of patients, although they should nonetheless be sent to referral centres. Unfortunately, the study showed that perforations occurred in three patients undergoing EMR-cap resection and in four patients undergoing MBM, but this rate is unusual, as can be seen in the table below.
 Question: What is your preference between the two techniques for removing tissue from the oesophagus: cap resection or MBM?
Question: What is your preference between the two techniques for removing tissue from the oesophagus: cap resection or MBM?
Answer: I started with cap resection so am used to this method and will continue to use it with most of my patients because of the experience gained, but I think the risk of perforation should be lower with MBM.
A historic paper analysing the result of mucosectomy in patients with low-risk early adenocarcinoma lesions (I, IIa, IIb and IIc), in which histological assessment showed no lymphatic or vein invasion, demonstrated that complete resection was achieved in 96% of cases (Gut 2008, 57:1200–06). Endoscopic resection failure occurred in 4% of patients, resulting in their having surgery, and metachronous lesions were seen in 21%. However, the five-year survival rate was very good, at 84%. If you are able to select patients carefully, I think that the five-year survival could be almost 100%.
What about cases where there is minimal infiltration of the submucosa? A very limited study suggests that in the most favourable cases – invasion of only the superficial layer of the submucosa, no infiltration of the lymphatic vessels or veins, a good histological differentiation grade G1 or G2, and macroscopic type I or II – then maybe oesophagectomy should be avoided (Am J Gastro-enterol 2008, 103:2589–97). However, when there is oesophageal invasion and the patient is fit for surgery, they should be referred to a surgeon.
Question: This can be quite a difficult diagnosis for a pathologist to make. What kind of pathologist should look at this type of tissue? And can you comment on how tissue should be delivered to the pathologist and how it should be processed in the laboratory?
Answer: This is a very interesting question. First of all, after each resection with EMR-cap you can immediately retrieve the specimen, which should be fixed by pinning it on a tablet. If you put it in a bottle it will shrink, making it difficult for the pathologist to make an adequate assessment, particularly of the depth of the lesion. You need to fix it as soon as you can. There are artefacts due to the resection and cauterisation, but in most cases we can achieve quite a deep resection. The pathologist should be trained in this area. There are a lot of problems in differentiating low-grade dysplasia from inflammatory reactions, and so on. It is always good to have a couple of pathologists judging a specimen.
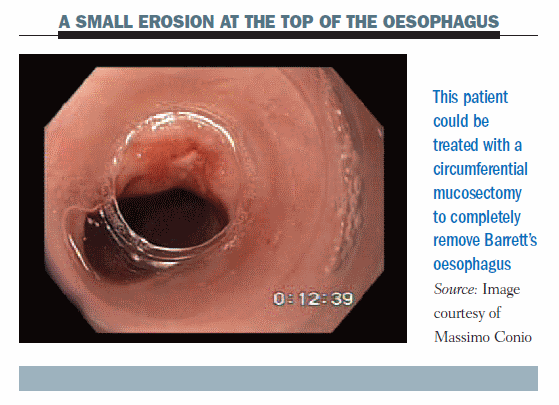
The figure below shows a patient with a small erosion at the top of the oesophagus. We decided to completely remove Barrett’s oesophagus, performing a circumferential mucosectomy.
 The figure below shows a patient with metachronous lesions, whom we decided to send to the surgeon. The bad news was that the patient underwent an oesophagectomy, but the good news was that there was no remaining cancer and the lymph nodes were negative.
The figure below shows a patient with metachronous lesions, whom we decided to send to the surgeon. The bad news was that the patient underwent an oesophagectomy, but the good news was that there was no remaining cancer and the lymph nodes were negative.
 Metachronous lesions after EMR
Metachronous lesions after EMR
There is a 30% risk of developing metachronous lesions after endoscopic mucosal resection. It has therefore been suggested that we should ensure complete eradication of Barrett’s oesophagus with stepwise endoscopic mucosal resection or with the new methods of radiofrequency ablation.
A retrospective study of stepwise radical endoscopic resection in 169 patients from four referral centres, with a Barrett’s oesophagus length <5 cm and endoscopic mucosal resection performed every four to eight weeks until complete eradication of Barrett’s oesophagus, showed eradication of neoplasia was obtained in 98% of patients, but eradication of intestinal metaplasia occurred in only 85% (Gut 2010, 59:1169–77). Four perforations occurred acutely during the procedure, and two perforations occurred late during dilation of stenoses. The main drawback of this technique is the onset of stenosis, which can be a real problem. In the study it affected 50% of patients.
Question: Does endoscopic mucosal resection become more difficult when you do this in a second procedure or in a third procedure? Is it more difficult to complete the whole resection if you come back a second or third time, because fibrosis could make it more difficult to lift and remove the lesion?
Answer: This is a problem, so in selective cases I prefer to carry out complete resection in one session only, and not stepwise, because of the risk that fibrosis due to scarring may prevent an adequate resection of the residual metaplastic tissue in the following endoscopic sessions.
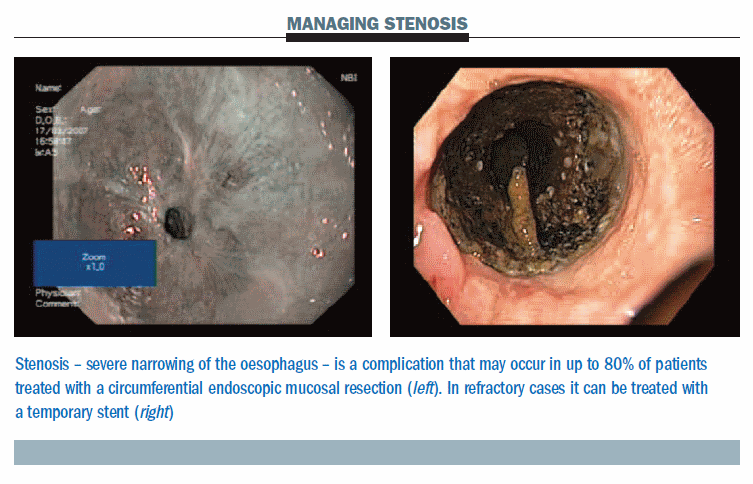
The figure below shows a significant stenosis occurring two weeks after a procedure (left). In refractory cases we use a removable stent (right), left in place for four to six weeks, which is easy to remove. We do not usually use stents larger than 16 mm. For the future, stents are being developed that will reduce the risk of migration, which can occur in these patients.
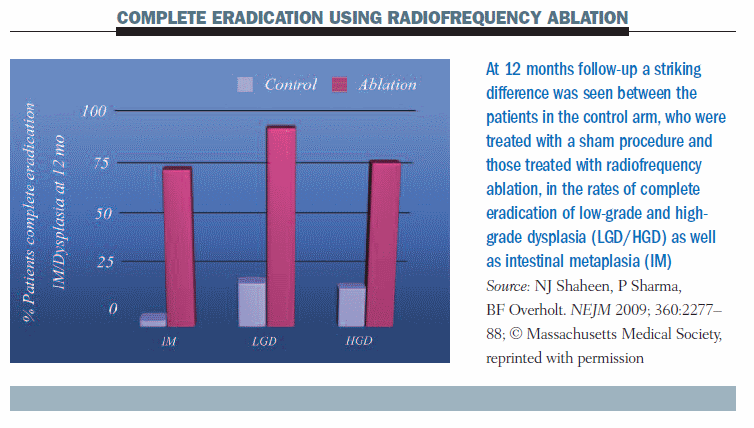
 The BARRX system can be used to achieve destruction of the oesophageal wall, using radiofrequency ablation with probes of 360o or 90o, until the muscularis submucosa. The stenosis rate with this system seems to be lower. A study by Shaheen published in 2009 (NEJM 360:2277–88) randomised 127 patients (64 with low-grade dysplasia, 63 with high-grade dysplasia) to radiofrequency ablation or a sham procedure. Complete eradication of intestinal metaplasia occurred in about 75% of patients with radiofrequency ablation at 12 months (see figure below); eradication of low-grade dysplasia was achieved in about 90% of patients and high-grade dysplasia in 80%. There was a striking difference compared to patients who did not receive this treatment. However, follow-up was relatively short, at only 12 months, and there was a problem with subsquamous intestinal metaplasia. The number of strictures was very small, however, at 6%.
The BARRX system can be used to achieve destruction of the oesophageal wall, using radiofrequency ablation with probes of 360o or 90o, until the muscularis submucosa. The stenosis rate with this system seems to be lower. A study by Shaheen published in 2009 (NEJM 360:2277–88) randomised 127 patients (64 with low-grade dysplasia, 63 with high-grade dysplasia) to radiofrequency ablation or a sham procedure. Complete eradication of intestinal metaplasia occurred in about 75% of patients with radiofrequency ablation at 12 months (see figure below); eradication of low-grade dysplasia was achieved in about 90% of patients and high-grade dysplasia in 80%. There was a striking difference compared to patients who did not receive this treatment. However, follow-up was relatively short, at only 12 months, and there was a problem with subsquamous intestinal metaplasia. The number of strictures was very small, however, at 6%.
 Question: In terms of the complete eradication rate, 75% may represent a good success, but on the other hand it means that 25% of patients have Barrett’s oesophagus left behind. This is not good because of the risk of developing malignancy.
Question: In terms of the complete eradication rate, 75% may represent a good success, but on the other hand it means that 25% of patients have Barrett’s oesophagus left behind. This is not good because of the risk of developing malignancy.
Answer: I think that many things must be analysed in patients undergoing radiofrequency ablation and we should, of course, achieve higher rates of eradication of intestinal metaplasia. There is always the risk of subsquamous mucosa and patients need to be maintained on a surveillance protocol.
The same group analysed the durability of response to radiofrequency ablation in a group of 106 patients with Barrett’s oesophagus with dysplasia. They aimed to evaluate the eradication of neoplasia; the eradication of Barrett’s oesophagus; the durability of response; disease progression and adverse events. The results for eradication of neoplasia and Barrett’s oesophagus at two years (n=106) were very good – 95% and 93%, respectively – and more or less the same at three years (n=56)– 98% and 91%, respectively (Gastroenterology 2011, 141:460–468).
At three years without maintenance, complete eradication of neoplasia occurred in more than 85% of patients and complete eradication of intestinal metaplasia in more than 75%. There is always the risk of persistence of metaplasia. Adverse events were reported in 3.4% of cases; oesophageal strictures accounted for 7.6% of adverse events.
In terms of disease progression, three patients with low-grade dysplasia progressed to high-grade dysplasia, one progressed from high-grade dysplasia to cancer, and one from low-grade dysplasia to adenocarcinoma. This rate means that 4.2% of patients must undergo endoscopic follow-up (one per 73 patient-years). The cost of surveillance of these patients will be maintained, but it still represents a great advance.
In an editorial accompanying the paper by Shaheen and colleagues, Inadomi pointed out that you need four radiofrequency ablation sessions to obtain complete eradication, and then 50% of these patients require further radiofrequency ablation sessions in the second and third year (Gastroenterology 2011, 141:417–419). This indicates we should use the term ‘remission’ and not ‘complete healing’ or ‘complete regression’, because there is always the risk of subsquamous persistence, even if it is minimal, which means we need to follow up.
 A multicentre randomised trial compared stepwise endoscopic mucosal resection (SEMR) against radiofrequency ablation (RFA) with or without endoscopic mucosal resection to look at the recurrence of stricture. It inclu-ded patients with Barrett’s oesophagus length <5 cm who underwent at least four sessions of SEMR or RFA following resection of focally resectable visible lesions (Gut 2011, 60:765–773). The study was relatively small, including only 47 patients (25 undergoing SEMR; 22 RFA±EMR). At the end of the follow-up, complete response of neoplasia occurred in 100% of patients in both arms, and complete response for intestinal metaplasia occurred in 20 out of 25 patients undergoing SEMR and in 18 out of 20 treated with RFA±EMR. But we are waiting for more results, because the number of patients is small and follow-up was only three years in the study mentioned previously, so we have to see what will happen in another two or three years before making decisions on treating every Barrett’s oesophagus patient with radiofrequency ablation.
A multicentre randomised trial compared stepwise endoscopic mucosal resection (SEMR) against radiofrequency ablation (RFA) with or without endoscopic mucosal resection to look at the recurrence of stricture. It inclu-ded patients with Barrett’s oesophagus length <5 cm who underwent at least four sessions of SEMR or RFA following resection of focally resectable visible lesions (Gut 2011, 60:765–773). The study was relatively small, including only 47 patients (25 undergoing SEMR; 22 RFA±EMR). At the end of the follow-up, complete response of neoplasia occurred in 100% of patients in both arms, and complete response for intestinal metaplasia occurred in 20 out of 25 patients undergoing SEMR and in 18 out of 20 treated with RFA±EMR. But we are waiting for more results, because the number of patients is small and follow-up was only three years in the study mentioned previously, so we have to see what will happen in another two or three years before making decisions on treating every Barrett’s oesophagus patient with radiofrequency ablation.
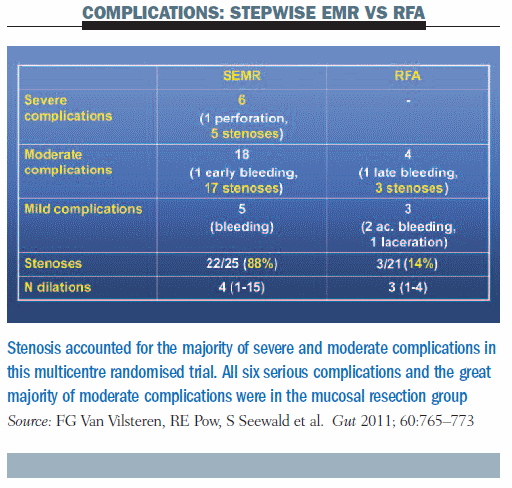
What about complications? The most important complications were stenoses: in the SEMR group, stenoses accounted for five of the six severe complications and for 17 of the moderate complications; in the RFA group, three stenoses occurred in patients with moderate complications. Overall, the rate of stenosis was 88% in the group undergoing mucosal resection versus 14% in the radiofrequency ablation group (see table below).
Based on current evidence, radiofrequency ablation should be offered to patients with low-grade and high-grade dysplasia and a long extension of Barrett’s oesophagus, because there is a low rate of stenosis and it is very easy to perform. Unfortunately, there is a lack of histological examination and we need to follow up.
Personal experience of one-step circumferential EMR-cap resection
I have just submitted for publication my group’s experience of one-step circumferential EMR-cap resection of Barrett’s oesophagus with early neoplasia. The study includes 47 patients with Barrett’s oesophageal length of about 3.0±1.4 cm, all with either high-grade dysplasia or intramucosal carcinoma (Conio et al, submitted 2012). Results showed a stenosis rate of 30% using one-step EMR in this group of patients. This is a significant rate of stenosis, but we should not worry about this if the procedure is performed at a centre where there is an expert and use of stents is routine, and we need to explain this to patients. Complete eradication of neoplasia and metaplasia was achieved in 96% of patients.
Of the 47 patients treated with circumferential endoscopic mucosal resection, only two underwent surgery: one took a personal decision to undergo oesophagectomy and the other underwent superficial adenocarcinoma. During follow-up of the remaining 45, seven (16%) had residual areas of Barrett’s oesophagus, which were very small (<5 mm) and were successfully treated with endoscopic ablation using argon plasma coagulation (APC).
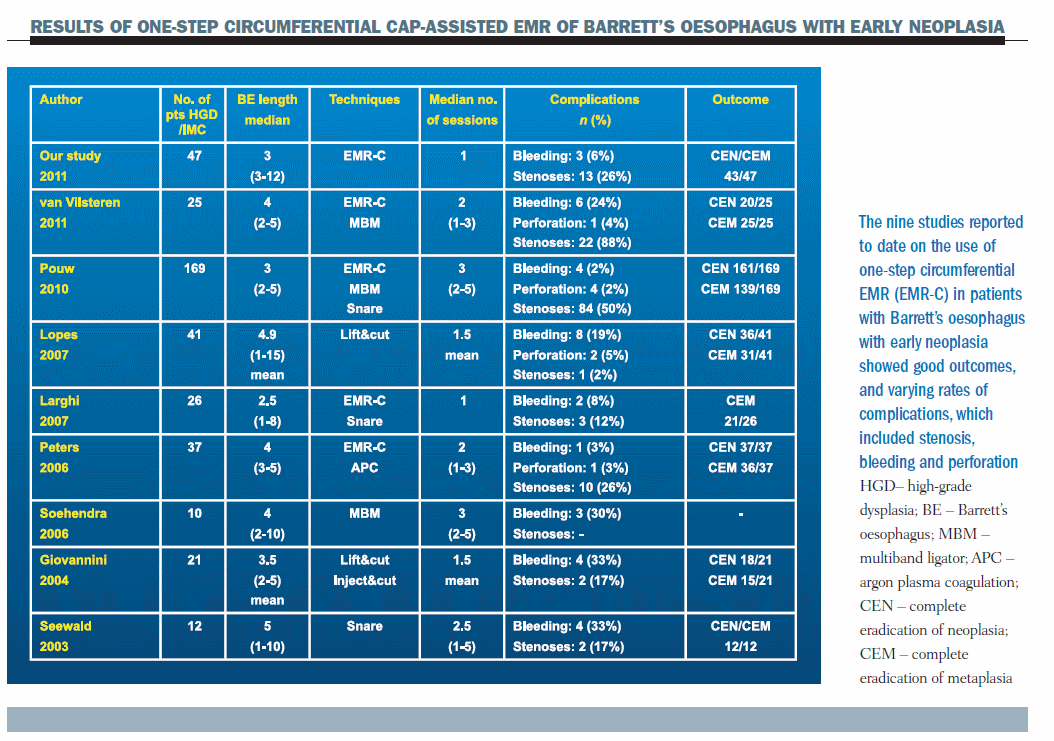
Overall, studies of one-step circumferential EMR-cap resection in Barrett’s oesophagus with early neoplasia show good outcomes (see table ‘Results of One-Step Circumferential Cap-assisted EMR of Barrett’s Oesophagus with Early Neoplasia,’ above). The main complication is stenosis, which affects between 2% and 88% of patients in the different studies.
Massimo Conio (MC): What do you think about risk of stenosis?
Peter Siersema (PS): Stenosis is one of the concerns with this technique. On the other hand, as shown clearly in your data, patients undergo a median of only one dilation session, so the majority of these strictures can be managed very easily in one or two dilation sessions. In my experience, stent placement is generally not required for dilation of these restrictions. So risk of stenosis is a cause for concern, but it is a manageable concern that does not affect your patients’ quality of life. Bleeding can be a concern, however, if you are not experienced. It seems quite severe and quite dramatic, but in my experience most of these bleedings can be managed endoscopically very easily with all the devices we have nowadays. MC: I agree. When you perform this technique you must be able to cope with any complications. The most feared complication is perforation, which almost never occurs, and bleeding. We can stop the bleeding quite easily and well with new hot biopsy forceps and it is not usually necessary to use clips. I had an experience three months ago in a patient with a long section of Barrett’s oesophagus who had torrential bleeding, and I was unable to pass any device through the channel. Because of high outflow of the blood I decided to tamponade by placing a Blakemore–Sengstaken tube, and I left it there for 24 hours. This was a first time for me, but the bleeding stopped after 24 hours. I think that this type of treatment should be performed only in referral centres where endoscopy experts are available. You must have every kind of device, and you must have the experience to cope with severe complications.
Managing perforations
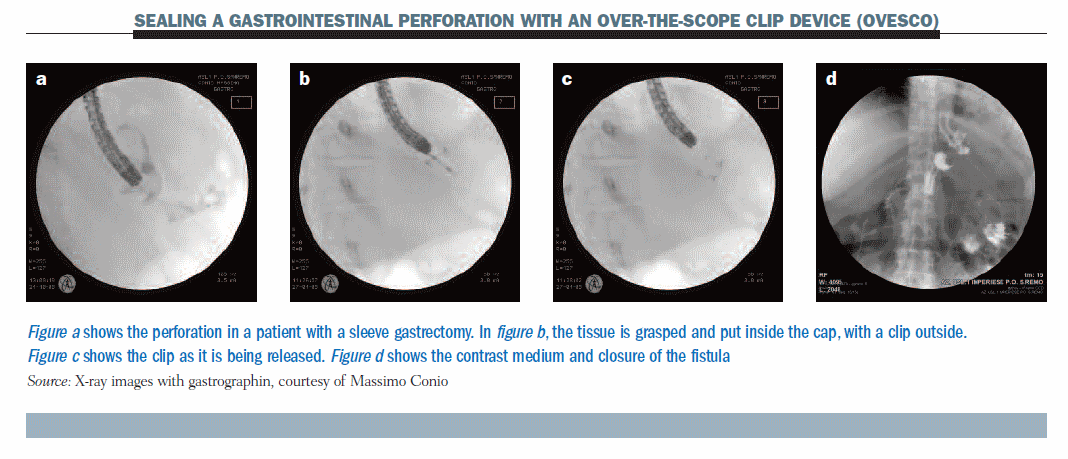
The figure below shows a new method which allows the sealing of gastrointestinal perforation with an over-the-scope clip device (OVESCO). (Details can be found in Gastrointest Endosc 2010, 72:881–886, with videos.)
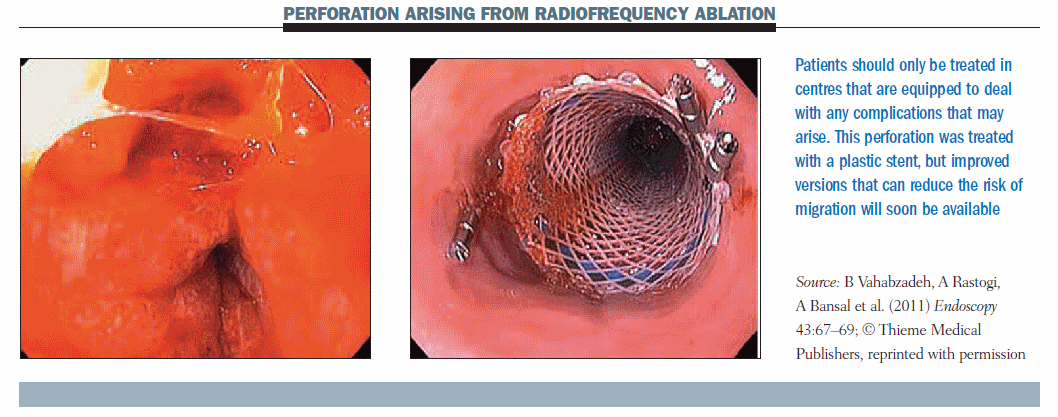
 The figure below shows an oesophageal perforation that occurred during radiofrequency ablation (Endoscopy 2011, 43:67–69). This corroborates my point that this procedure should only be performed in a centre that has all available instruments. This case was treated with removable plastic stents, but nowadays I think we can afford to use other stents.
The figure below shows an oesophageal perforation that occurred during radiofrequency ablation (Endoscopy 2011, 43:67–69). This corroborates my point that this procedure should only be performed in a centre that has all available instruments. This case was treated with removable plastic stents, but nowadays I think we can afford to use other stents.
 In a patient with Barrett’s oesophagus, high-grade dysplasia and intramucosal cancer, we perform endoscopic ultrasonography, mainly for the evaluation of the lymph nodes. But if is quite short (C ≤ 4 cm, using the Prague classification) we can probably treat the patient with endoscopic mucosal resection. If it is longer, I would suggest it is better to perform EMR focally plus HALO radiofrequency ablation. The histology will determine the patient’s further treatment. If there are no dysplasia or everything is confined above the muscularis mucosa, we can offer surveillance. But surveillance should probably stop after a couple of years if we have removed everything. This approach has not yet been proved for patients with submucosal cancer, and we have to send them to surgery.
In a patient with Barrett’s oesophagus, high-grade dysplasia and intramucosal cancer, we perform endoscopic ultrasonography, mainly for the evaluation of the lymph nodes. But if is quite short (C ≤ 4 cm, using the Prague classification) we can probably treat the patient with endoscopic mucosal resection. If it is longer, I would suggest it is better to perform EMR focally plus HALO radiofrequency ablation. The histology will determine the patient’s further treatment. If there are no dysplasia or everything is confined above the muscularis mucosa, we can offer surveillance. But surveillance should probably stop after a couple of years if we have removed everything. This approach has not yet been proved for patients with submucosal cancer, and we have to send them to surgery.
Question: What is the next step of follow-up after EMR in your study?
Answer: I have not provided all the data from my studies, but we did not find cancer or dysplastic lesions after two years of follow-up. So we suggest that in patients who had complete resection, follow-up should be carried out every five years rather than two years. There are other issues, such as what happened at the level of the new squamous/columnar juncture, because it has been shown that a lower- grade dysplasia might be found. But I would say that complete resection may allow us to observe these patients every five years after the first year of intense control.
A study on the risk of progression in patients with non-dysplastic Barrett’s oesophagus showed that 96% of patients followed for five years did not develop cancer; after 10 years, 97% of this group had still not developed cancer (Clin Gastroenterol Hepatol 2011, 9:220–227). The risk of progression was very small, and was only in patients with longer extension (0.65% per year in patients with a Barrett’s oesophagus measuring ≥ 6 cm). In my opinion, this means that radiofrequency ablation should be the preferred option in all patients who have a long extension, even if they have no form of dysplasia, because it is very difficult to sample everything.
MC: A Danish study published recently in the New England Journal of Medicine (2011, 365:1375–1383) of more than 11,000 Barrett’s patients showed the risk of disease progression was very low. So it is a nonsense to recommend radiofrequency ablation in every patient with Barrett’s oesophagus that is not complicated even by low-grade dysplasia. What do you think?
PS: I do think that there might be a question for patients with low-grade dysplasia in the long term, especially now we know some more long-term results on this. But there is now a tendency in the US for people to start treating patients without any dysplasia. We know that the risk in that group is very low and there is no justification for treatment with radiofrequency ablation. I think for low-grade dysplasia it is still too early, and in recognised and characterised low-grade dysplasia there might be a case for ablation therapy.
Question: Looking at your treatment algorithm for high-grade dysplasia and intramucosal cancer (see below), you performed EMR or EMR+HALO RFA. When histology showed no dysplasia you performed surveillance, and for invasive cancer you performed surgery. I agree, but I do not fully understand what you mean for patients with high-grade dysplasia and early cancer who then undergo surveillance. Is that not something that you need to treat?
Answer: If histology detected intra-epithelial neoplasia, then the patient should undergo endoscopic follow-up for at least a couple of years. I am talking about patients with radical removal of intestinal metaplasia in Barrett’s oesophagus. In the future, we should avoid performing focal mucosal resection because it does not make sense. The risk of metachronous lesions is very high. Insert Figure 10 here.
Summing up
Radiofrequency ablation is a very promising technique, but we are still waiting for long-term results after three years. There is plenty of room for further randomised studies. I would suggest these should ideally be pan-European studies, because this is quite a rare disease and not many centres are able to recruit a large number of these patients. A high level of endoscopic expertise is required and patients should be sent to referral centres that have a wide range of multimodality treatments.
PS: While chairing a session on Barrett’s oesophagus for young gastroenterologists at the recent UEGW (United European Gastroenterology Week), I found that it is sometimes difficult for gastroenterologists to convince their surgeons that patients with early cancers or high-grade dysplasia should undergo endoscopic treatment and not surgical treatment. How would you advise these gastroenterologists to convince their surgeons that it’s really worthwhile to consider endoscopic treatment in these patients instead of performing an open resection, which is still being done in quite a few centres all over Europe?
MC: The most important evidence is the literature. The data have shown that the results are comparable. In the best centres in the world, the mortality rate for oesophagectomy is 3% and the morbidity rate is about 40%. Surgeons should understand we are not competitors – we have to work together. If there is a patient in whom mucosectomy shows an invasive cancer infiltrating the muscularis mucosa – even the upper layer – surgery should be carried out. But the quality of life with endoscopic treatment versus surgery is very different. Even the first day after endoscopic treatment, the patient feels very well, with no pain, and can go home in 48 hours. In contrast, there are a range of complications with surgery, including anastomosis leakage and impaired ability to eat normally.
The European School of Oncology presents weekly e-grandrounds which offer participants the opportunity to discuss a range of cutting-edge issues, from controversial areas and the latest scientific developments to challenging clinical cases, with leading European experts in the field. One of these is selected for publication in each issue of Cancer World. In this issue, Massimo Conio, of the Department of Gastroenterology, Sanremo Hospital, Sanremo, Italy, provides an update on the latest developments in the use of endoscopic treatment in patients with Barrett’s oesophagus complicated by superficial cancer. Peter Siersema, of the University Medical Centre Utrecht, in the Netherlands, poses questions arising during the e-grandround live presentation. The presentation is summarised by Susan Mayor.



Leave a Reply