Targeted drugs can result in a variety of skin toxicities that are unpleasant for patients and, if unattended to, can lead them to stop taking their drug as prescribed. Effective teamwork is required to ensure symptoms are identified and managed.
 The European School of Oncology presents weekly e-grandrounds which offer participants the chance to discuss a range of cutting-edge issues with leading European experts. One of these is selected for publication in each issue of Cancer World.
The European School of Oncology presents weekly e-grandrounds which offer participants the chance to discuss a range of cutting-edge issues with leading European experts. One of these is selected for publication in each issue of Cancer World.
In this issue Christine Boers-Doets, from the department of Clinical Oncology at Leiden University Medical Centre in The Netherlands, reviews the occurrence and management of skin toxicities caused by targeted therapies. Annie Young, from the University of Warwick, in Coventry, UK, poses questions arising during the e-grandround live presentation.
Written by Susan Mayor.
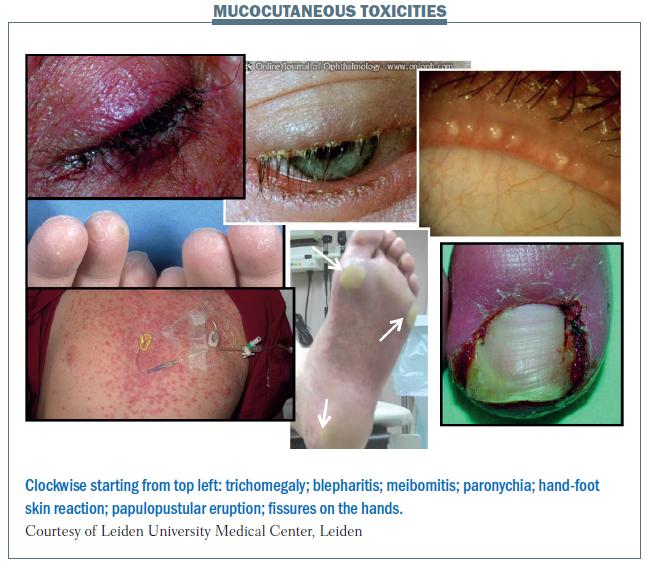
Mucocutaneous adverse events are relatively common with targeted agents, and there is a relationship between skin toxicities and side-effects as a whole. Papulopustular eruption is the commonest skin toxicity, with dry skin, itchy skin and hand-foot skin reaction also being common. The figure below illustrates some of the mucocutaneous reactions that occur with targeted agents. These include trichomegaly (long lashes), which can occur with EGFR inhibitors, and blepharitis (inflammation of the rims of the eyelids), which is a common side-effect with EGFR inhibitors, but has not been reported very widely in the literature because no-one is looking for it carefully. The connection between skin toxicities and mucosal toxicities is illustrated by side-effects affecting both the eyelid (skin) and eye (mucocutaneous tissue).
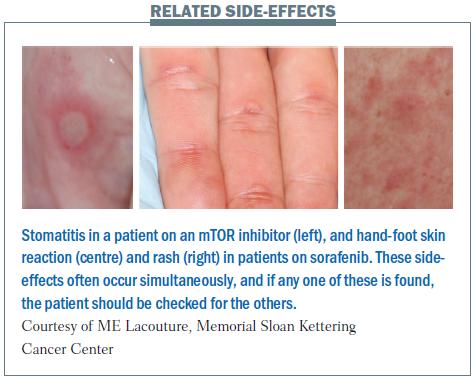
 There is a correlation between stomatitis (inflammation of the mucosal lining in the mouth), hand-foot skin reaction and rash. Their appearance is similar, with round ulceration and an erythematous halo (see figure below), underlining the connection between these side-effects, which can occur with targeted agents.
There is a correlation between stomatitis (inflammation of the mucosal lining in the mouth), hand-foot skin reaction and rash. Their appearance is similar, with round ulceration and an erythematous halo (see figure below), underlining the connection between these side-effects, which can occur with targeted agents.
 The connection between hand-foot skin reaction and stomatitis is illustrated in a study of patients treated with sunitinib: of the 13 patients who had grade 3 hand-foot skin reaction, 10 also had stomatitis (Br J Dermatol 2009, 151:1045–51). Of the 76 patients who did not have hand-foot skin reaction, only 18 had stomatitis. This tells us that if you see severe stomatitis or severe hand-foot skin reaction in a patient, you should look at their feet or in their mouth to see whether there are other mucocutaneous side-effects. The same connection between cutaneous side-effects and stomatitis occurs with mTOR inhibitors (Cancer 2010, 116:210–215).
The connection between hand-foot skin reaction and stomatitis is illustrated in a study of patients treated with sunitinib: of the 13 patients who had grade 3 hand-foot skin reaction, 10 also had stomatitis (Br J Dermatol 2009, 151:1045–51). Of the 76 patients who did not have hand-foot skin reaction, only 18 had stomatitis. This tells us that if you see severe stomatitis or severe hand-foot skin reaction in a patient, you should look at their feet or in their mouth to see whether there are other mucocutaneous side-effects. The same connection between cutaneous side-effects and stomatitis occurs with mTOR inhibitors (Cancer 2010, 116:210–215).
AY: Do all agents have similar side-effects?
CB-D: There are differences between the agents. When side-effects occur, the appearance is basically the same, but the grade and duration is different. There is less of a rash with mTOR inhibitors, but the appearance is the same as with other agents and treatment is the same.
AY: You mentioned the relationship between the mucosa and dermatological toxicities; do you have any idea why the pathology underlying this relationship makes them occur together?
CB-D: It is because the agents bind to receptors in the skin and mucosa as well, so the side-effects develop at both these locations.
AY: Do you think that you will see the same relationship between mucosal and skin toxicities with EGFR inhibitors?
CB-D: Yes. I did not find evidence for this correlation in the literature, but we see it very often in daily practice. It is important to ask the patients, because the focus tends to be on rash and paronychia, while there is very little awareness about other side-effects.
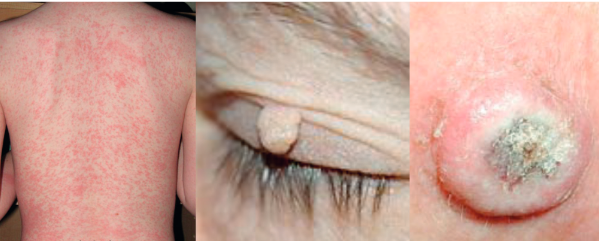
Papulopustular rash
This was previously termed acneiform rash or eruption, but it is now known as a papulopustular rash, because patients have papules (small raised pimples) and pustules (small pus-filled blisters). When patients just have papules, the rash appears red, while pustules appear yellow. The picture of the nose in the upper part of the bottom figure opposite shows a crust developing, which is a good sign, because it means that the rash is fading and the patient can continue therapy.
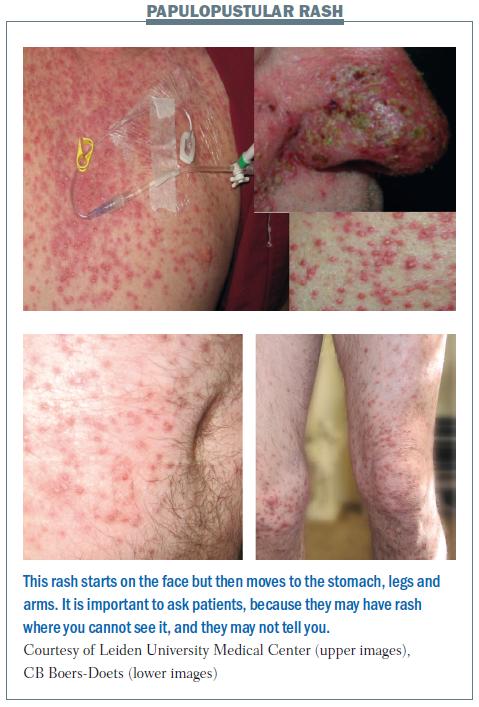 Papulopustular rash first appears on the face. Later it disappears from the face and scalp, but occurs on the stomach, legs and arms. The patient in the lower part of the figure did not tell us that he had a rash. We did not see anything on his face and he did not tell us about the rash on his body and legs, so we nearly missed it. He only told us when we noticed that he had difficulty in walking and sitting down and we asked why. Treatment of symptoms should be tetracycline twice a day.
Papulopustular rash first appears on the face. Later it disappears from the face and scalp, but occurs on the stomach, legs and arms. The patient in the lower part of the figure did not tell us that he had a rash. We did not see anything on his face and he did not tell us about the rash on his body and legs, so we nearly missed it. He only told us when we noticed that he had difficulty in walking and sitting down and we asked why. Treatment of symptoms should be tetracycline twice a day.
The best evidence we have today indicates that tetracycline should also be used prophylactically (the STEPP trial, JCO 2010, 28:1351–57). The limitation of this study is that tetracycline was used in combination with sunscreen and topical steroid, so we do not know whether the effect was due to tetracycline alone or whether the sunscreen and topical steroid also played a role. Other treatments being trialled for treatment of skin rash include vitamins K1 and K3, sun protection and pro-vitamin B5 (Bepanthen cream).
AY: A team from Belgium uses doxycycline 100 mg once a day. Is that alright for the management of skin rash?
CB-D: The literature says 100 mg once or twice a day given prophylactically is required. In my opinion, and the opinion of members of MASCC [Multinational Association of Supportive Care in Cancer], 100 mg once a day is enough for prophylactic use. However, when you are managing side-effects you need to increase the dose to 200 mg. If there is no response after 14 days, take swabs and check for resistance and prescribe antibiotics based on culture results.
AY: Should vitamin K ointment be used prophylactically for rash?
CB-D: I am performing a Cochrane review on this topic, so I have read all the publications on it. We assessed which products help and which do not. When we put the information together, we only know that we have to use an ointment to reduce rash. Results are conflicting for the use of vitamin K ointment and we need more studies to gain further information. I think the problem is that the right tools were not available to assess the benefit.
AY: Do you have any experience with any vitamin K ointments or treatments?
CB-D: Here in The Netherlands we give them to patients treated with cetuximab. I am performing a study with pro-vitamin B5 (dexpanthenol) with 160 patients. We will find out whether we can reduce antibiotics, because these have side-effects too. Results with vitamin K1 and pro-vitamin B5 are positive so far, but we need more data.
BRAF-specific skin reactions
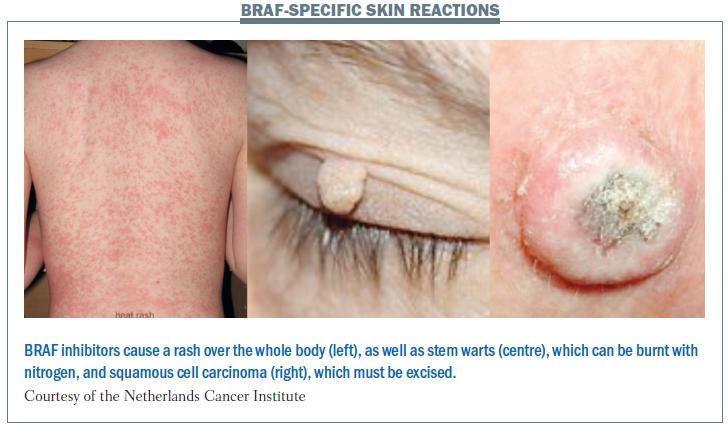
There are a greater number of side-effects with BRAF inhibitors, including some new types of side-effects (see figure below). The rash is similar to that seen with other targeted agents, but the whole body is affected. Patients may develop stem warts, which can be burnt off with nitrogen. Squamous cell carcinoma is a very common side-effect with vemurafenib, which is used against BRAF-positive melanoma. In addition to these unique side-effects, patients on BRAF inhibitors may also suffer side-effects associated with other targeted agents, such as hand-foot skin reaction and sensitivity to sunlight.
 Dry skin
Dry skin
Dry skin (xerosis cutis) is a very common side-effect with targeted agents, and is associated with many other skintoxicities such as rash, photosensitivity, scaling of the skin, fissures and hand-foot skin reaction. To prevent dryness and rash, cleansing with mild soaps and moisturising twice daily with thick, emollient creams is recommended (Clin J Oncol Nurs 2008, 12:283–290). Patients should be advised to avoid prolonged hot showers and use only products that are alcohol-, fragrance- and dye-free (Oncol Nurs Forum 2008, 35:103–111). Cool or lukewarm water should be used for bathing or washing, and moisturisers should be applied immediately afterwards. In addition, patients need to stay hydrated by drinking plenty of fluids.
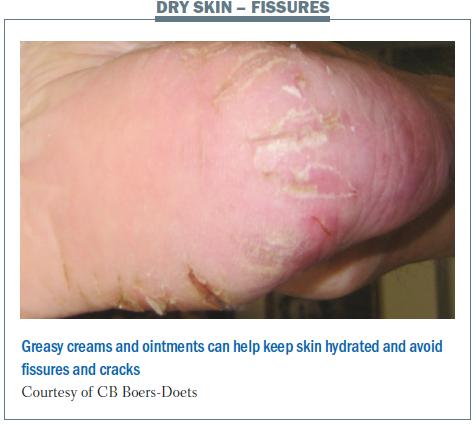
Patients may also develop flakes on the skin, which are itchy and, if severe, can be removed by treatment with salicylic acid. Patients with dry skin may also have fissures, which result in cracking. This can be treated with greasy creams and ointments.
 AY: Which cream or ointment is optimal for patients?
AY: Which cream or ointment is optimal for patients?
CB-D: You need a greasy cream or ointment in a pot. The others are too watery and they will not hydrate enough.
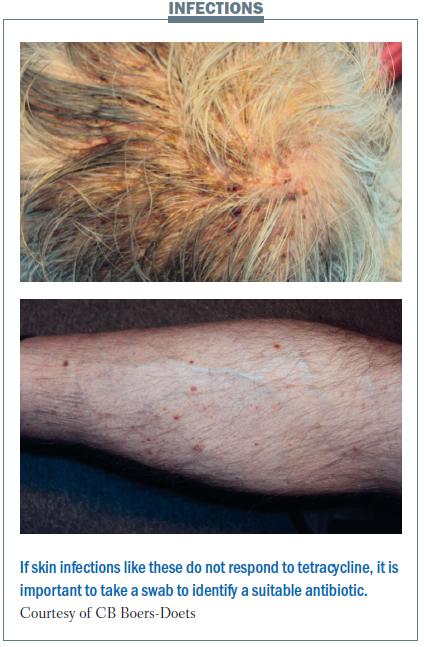
Skin infections
The pain from skin infection can be very severe. The patient with scalp infection shown in the figure below could not take showers or comb his hair because of his painful scalp. He was treated with tetracycline, but this did not help. Taking a culture showed his infection was resistant to tetracycline, so treatment was changed to another antibiotic. The scalp infection was cured, but the infection reappeared on the patient’s leg. Taking a swab showed he had Staphylococcus aureus infection that was resistant to clindamycin, erythromycin and tetracycline. An antibiotic was chosen based on the sensitivity results. Before we learned about looking at the sensitivity to the antibiotics, we used to increase the dose of tetracycline or add topical corticosteroids, but that seldom resolved the skin side-effects.
Nail fold changes
Nail fold changes (paronychia) are very common side-effects that affect the fingers or toes. Paronychia is very painful, and patients whose toes are affected cannot wear shoes or socks, and therefore have to wear slippers. It can become infected, which appears as redness.
The figure below shows a case of paronychia with pyogenic granuloma (an overgrowth of tissue). A dermatologist may identify this incorrectly as an ingrown nail and try to remove the nail, but this is not an appropriate management option, since the skin overgrowth can be managed by patients themselves.
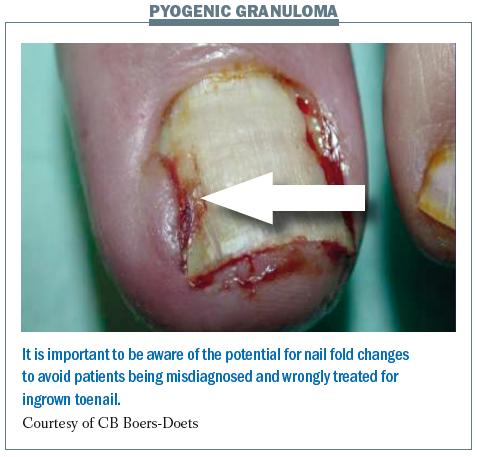 We recommend that patients with nail fold changes bathe affected nails with table vinegar and water (1:1) twice a day for 15–30 minutes. This will help to reduce the paronychia. In the case of ulceration, or if there is evidence of the situation becoming more severe, a culture is needed to find out if it is infected. It should be checked for antibiotic resistance to guide antibiotic selection.
We recommend that patients with nail fold changes bathe affected nails with table vinegar and water (1:1) twice a day for 15–30 minutes. This will help to reduce the paronychia. In the case of ulceration, or if there is evidence of the situation becoming more severe, a culture is needed to find out if it is infected. It should be checked for antibiotic resistance to guide antibiotic selection.
When pyogenic granuloma is present, silver nitrate applicators can be used once or twice a week. Patients must be warned to apply treatment only to the granuloma and not to the nail or the nail fold, because it will stain them black.
Special challenges in the elderly
Due to ageing, elderly patients tend to have dry skin and mucosa when they begin therapy. This increases the risk of more severe dermatological side-effects, and more eye and oral problems. Older people also have an increased risk of skin trauma because they are more likely to slip, and this risk is increased further with hand-foot skin reaction. Neuropathy is another problem more common in older people; it can lead to worsening of fissures because it lowers awareness of skin cracking.
Measuring and managing skin toxicities
It is very important that patients with cancer receive optimal treatment with targeted agents, at the optimal dose and for the planned duration. There is a lot of literature showing rapid tumour growth in patients who stop treatment or who have a break for a few weeks, so reducing the dose is not a desirable option. It is therefore essential to avoid having to stop therapy because of skin reactions.
In general, younger patients have a lower overall health-related quality of life than older patients with the same adverse events. This means they are more likely to stop treatment early because of the side-effects, and it is important to avoid this happening (Cancer 2010, 116:3916–23).
The physical domain has the greatest impact on health-related quality of life. Patients with a rash have a lower health-related quality of life than patients without rash, primarily because of the burning sensation the rash causes. (JCO 2007, 25(18)S19532).
Patients may have multiple problems, and it is very important to enquire about them all. For example, most patients will not report skin or scalp bleeding unless they are asked. Most report skin bleeding when asked specifically, including skin bleeding on their bed sheets during the night, when they take showers and when they are changing their clothes. Skin bleeding is very distressing because it is painful, occurs frequently and it marks clothing.
Assessing health-related quality of life is very important, and can be done with a variety of tools.
Dermatology-specific health-related quality of life tools used in studies with targeted agents include the SKINDEX-62, -29 and -16, which is the most commonly used, and the Dermatology Life Quality Index (DLQI).
Health-related quality of life tools have been developed for use specifically in patients prescribed EGFR-inhibitors, based on the SKINDEX-16. The Functional Assessment of Side-effects to Therapy–EGFRI (FAST-EGFRI-38) had 38 questions and was too long. A shorter version was therefore compiled – the Functional Assessment of Cancer Therapy– EGFRI-18 (FACT–EGFRI-18), with 18 questions, which is a good questionnaire to use.
It is a patient-reported outcome questionnaire that consists of 18 items arranged in three health-related quality of life dimensions: physical (7 questions); social and emotional (6 questions) and functional wellbeing (5 questions). It is important to assess all three domains. The questionnaire is very easy for patients to use, with a five-point Likert-type response scale. It assesses only skin, nail and hair side-effects, and does not include mucosal side-effects, so questions need to be added to cover this area.
FACT-EGFRI-18 was developed in the USA in English. We are carrying out a clinical trial with this tool, so we have translated it into Dutch, and it is currently being formally validated after linguistic validation. It has also been translated into German, but we are looking for colleagues to carry out linguistic and formal validation.
In our linguistic validation we found that it was the symptom burden – and not cosmetic appearance – that affected health-related quality of life in the first month. Patients said the worst symptoms were increased sensitivity to sunlight, because of the burning sensation. Itching of the skin and scalp and bleeding of the skin were also major problems.
We need to focus on these side-effects because they interfere most with patients’ quality of life. Our results are consistent with a previous study in a similar group of patients (Oncology 2007, 21 S5:34–36), so both studies provide useful information to guide daily practice.
We are also evaluating the Vanderbilt Head and Neck Cancer Symptoms Survey (VHNSS2.0) for side-effects associated with mTOR inhibitors and mouth ulcerations associated with tyrosine kinase inhibitors.
AY: Which is the best tool to use in the clinic? Do they take a lot of time?
CB-D: The SKINDEX-16 is very short, with only 16 questions. It is translated into many languages, but there are different questions for different countries, so the data from different countries cannot be compared.
AY: Do you use the FACT–EGFRI-18 in practice in your clinics?
CB-D: Yes. I use it because I want to know which side-effect is most distressing for the patient, so we can focus on that.
AY: Can you do a symptom cluster? Can you assess all the symptoms as well as skin toxicities at the same time? Or are there different tools for different side-effects?
CB-D: The questionnaires we have now are only for side-effects with EGFR inhibitors, not for all targeted agents. In my opinion we need a broader questionnaire. Also they assess only the health-related quality of life and not the grade of side-effects, which means they do not assess symptom burden. We need another questionnaire for that.
Take home messages
It is very important to keep the skin hydrated in patients treated with targeted anticancer agents. When infected skin rash is suspected, start treatment with tetracycline, but if this does not help it is important to take swabs and select antibiotic therapy based on microbial sensitivity.