The need to give greater weight to patients’ own assessments of treatment impacts is increasingly accepted in principle. Putting it into practice will require a lot of hard work, developing tools that work for specific conditions and treatments, are easy to use, and command an international consensus. Simon Crompton talks to some of the people who are determined to make it happen.
The answer to the meaning of life, the universe and everything is 42, according to celebrated science fiction writer Douglas Adams. Roger Wilson, founder of Sarcoma UK and one of the most prominent cancer advocates in Europe, says he has found his 42: patient reported outcome measures.
He believes that these measuring tools of quality of life could put patient experience at the centre of research, clinical decision making and treatment availability – life, the universe, everything.
Patient reported outcome measures (PROMs) use patients’ own assessments of their quality of life, capturing subjective experience through questionnaires. They have long been used in clinical practice and research to monitor patients during treatment, looking to measure physical symptoms, psychological problems and general quality of life.
But their use is patchy, inconsistent and uncoordinated. Wilson – who has advised the UK’s National Cancer Director and was honoured in 2011 for services to healthcare – is on a mission to change that. He wants systematically gathered information about what gives patients a good quality of life to guide everything – research into drug treatments, clinical decision making, health technology assessments, cancer policy.

His vision is about to be spelled out in a far-reaching piece in the journal Research Involvement and Engagement – a rare example of a patient sole-authored paper in the peer-reviewed medical press. Wilson argues that the development of new cancer treatments is guided not by the value they add to patients’ lives but by convoluted surrogate endpoints. Equally, treatment choice is informed by clinician opinion rather than patients’ past experience of what works. Patient quality of life data, he says, must be standardised and gathered on a massive scale, so that whole pathways of care in every disease can be guided by what has actually helped patients live fulfilled lives.
“We need to measure and describe the pathways experienced by patients in terms that they understand,” he says. “This would be done by bringing together quality of life data from a range of clinical and research sources, and aggregating and analysing it, to describe stages in the disease pathway.”
Wilson’s own experiences of cancer over 18 years demonstrate current gaps – and the potential of PROMs to fill them. Since being diagnosed with soft tissue sarcoma in 1999, Wilson, a former producer at the UK broadcaster the BBC, has had ten operations, chemotherapy and radiotherapy. These included a lower leg amputation in 2007.
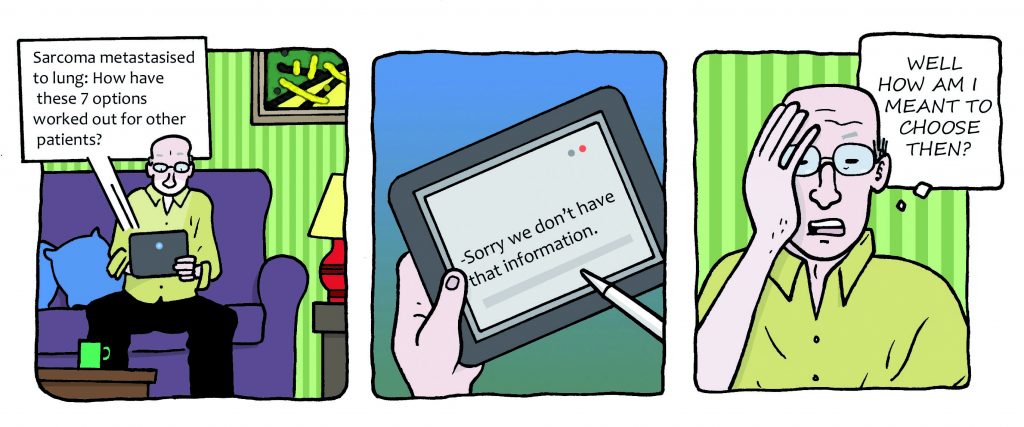
It was his experiences when diagnosed with lung metastases in 2013 that truly convinced him that a change had to come. He was presented with several options: surgery, different types of ablative therapy, chemotherapy and palliative care. But the right choice was far from clear. There was no evidence about outcomes from each option for someone in Wilson’s circumstances, and no quality of life data apart from one palliative care study. All he had to go on was clinical experience and informed opinion.
“I wanted the pathway which offered the fullest and longest life,
and currently the data isn’t there”
“I was at a branch point in my pathway,” says Wilson. “And there were between four and seven routes I could follow, and the chosen one would unfold as my pathway from that decision point. In my own non-curative situation, all the pathways available would probably collapse into one at some future point. But I wanted the pathway which offered the fullest and longest life, and currently the data isn’t there to inform the answer. Only information from patients can answer that question.”
Having taken the best advice he could, from all the contacts he had, Wilson opted for innovative laser knife surgery. But he acknowledges that if he had had more information about patient experience along each of the treatment pathways, he might have taken a different decision.
How might this be achieved? The vision is that patients’ own reports of quality of life are comprehensively recorded in every trial of every treatment and in every clinical intervention. This doesn’t just have benefits in terms of monitoring patients as they undergo treatment. It produces a vast pooled database of experience that can be used to assess outcomes and inform decision making at every point: drug approvals, clinical guidelines, treatment availability and health policy decisions.
“What I’m after, ultimately, is that anyone with a smart phone can report just one piece of data every day,” says Wilson. “They could be reporting on pain one day, fatigue another, psychological feelings another. And even if you had a rare disease such as sarcoma, 100 patients feeding back on surgery by a particular surgeon, or a particular treatment over a year, you get extremely useful feedback.”
Integrating PROMs into cancer care
It’s not just patient advocates who are enthusiastic about PROMs. The Centre for Patient Reported Outcomes Research at Birmingham University aims to optimise the use of PROMs in clinical trials and routine care, to improve outcomes and ensure that the patient perspective is at the heart of health research and decision making. Patient partners, including Roger Wilson, are closely involved in the work.
Melanie Calvert, Director of the centre, says PROM data should be integral to cancer care. “Introduction of PROMs into a healthcare system can have a number of benefits and has the potential to tailor care to individual patient needs.”
The immediate benefits in terms of monitoring patients are already clear. Recent work by Ethan Basch from the University of North Carolina shows that clinicians miss around half the symptoms experienced by chemotherapy patients. Using electronic systems where patients can continually record their quality of life allows a mechanism for early detection of symptoms and rapid response. Basch’s team has shown electronic PROM use reduces hospitalisation and improves survival.
A recent review of evidence by Cancer Care Ontario in Canada found that use of PROMs in routine cancer care is popular with patients, enables earlier detection of symptoms and aids communication between clinicians and patients. Many PROMS are already used for monitoring – for example PROMIS (Patient Reported Outcomes Measurement Information System) and the QLQ-C30 quality of life questionnaire, developed by the EORTC. The Ambuflex telehealth system of patients reporting their symptoms and life quality via online questionnaires has been widely implemented in Denmark.
“PROMs data should be integral to regulatory
and commissioning decisions”
But the potential benefits go way beyond monitoring. “In cancer care, patient reported outcome data collected in clinical trials can help future patients make informed choices,” says Calvert.
“In addition, the data can be used to inform clinical guidelines and health policy. In my opinion these data should be integral to decisions made by drug regulators and commissioners, alongside survival and safety data.”
Guiding approvals and access
Never has the need for this been clearer, as increasing evidence emerges that current decisions on treatment development and availability are skewed by commercial priorities rather than reflecting patient need. A systematic evaluation of oncology drug approvals by the European Medicines Agency (EMA) in 2009–13, published in the British Medical Journal last year, found that most drugs entered the market without evidence of benefit on survival or quality of life. Of 68 cancer indications with EMA approval, only 35 showed significant survival or quality of life improvement after three years.
An analysis of FDA cancer drug approvals in 2016, by Canadian researcher Christopher Booth in Nature Reviews Clinical Oncology last year, found that many approved agents offer only marginal value to patients, judged by the ESMO Magnitude of Clinical Benefit Scale.
Roger Wilson believes that drug approvals – and decisions about availability – are too far removed from patient experience. He points to research by Ian Tannock, presented to the National Cancer Research Institute in 2014, which reviewed major randomised controlled trials in breast, lung and colon cancer since 1975. He found evidence of smaller and smaller benefits from new drugs, researchers using complex surrogate endpoints, under-reporting of side effects and under-researching of quality of life.
“The analysis suggests that data are garnished to claim fancy conclusions, that a few weeks’ added life is hyped as significant benefit, and that data on outcomes that patients really worry about – like the day-to-day effect of their treatment – are missing,” says Wilson. He worries that the situation could get worse, as excitement about immunotherapy, pharmacogenomics and precision medicine threatens to obscure realities for patient – such as new types of side effect and the need for regular biopsies on relapse.
For patient advocates like Wilson, it’s part of a bigger picture of cursory patient involvement in running trials. For all the patient ‘representation’ on committees, how often do patient perspectives on quality of life guide assessment of outcome? If they are included as an endpoint, it tends to be secondary rather than primary.
Types of PROM
There are two main types of PROM, measuring di erent measures of quality of life:
Generic measures of quality of life
- enable easy comparisons across diseases,
- measure general functioning and quality of life over time.
The most commonly used generic measure is EQ-5D.
Disease-specific measures of quality of life
- are responsive and clinically useful
- measure frequency and severity of speci c symptoms (e.g. nausea, fatigue).
Some patient reported outcome surveys integrate questions measuring disease-speci c symptoms with questions measuring general quality of life. Together, generic and disease-specific PROM questionnaires allow patients to record both symptoms and their impact on their everyday functioning.
Slow progress
The University of Birmingham’s Centre for Patient Reported Outcomes Research says there is evidence that patient-reported quality of life information is often omitted, poorly collected or badly reported in trials. In a major new study called EPiC, funded by Macmillan Cancer Support, the centre has joined with international collaborators to investigate how well – or poorly – PROMs are being used in UK cancer trials. Lead researcher Derek Kyte says that if PROM data is not being effectively collected and reported, “it is less likely to effectively inform patient and clinician decision making at the point of diagnosis and beyond, and represents a waste of limited healthcare and research resources.”
One of the EPiC collaborators is Fabio Efficace, Head of the Health Outcomes Research Unit at Fondazione Gimema, Adjunct Professor at Northwestern University, Chicago, and Chair of the EORTC Quality of Life Group. He’s pleased that, over the past 20 years, more and more trials have included a PROM component, reflecting patient perspectives rather than solely physician views on adverse events.
“The major evidence we have now is that the adverse events reported by clinicians typically represent an underestimation of the real symptom burden perceived by the patient themselves,” he says. “Well-validated PROMs are the only way to translate the patient voice into clinically meaningful data that should better inform clinical decisions.”
The need is clear. But if Roger Wilson is to see his vision realised, a multitude of barriers and limitations need to be overcome.
Making PROMs usable
It isn’t just the problem of PROMs being poorly applied. There’s also the problem of making sure that patients regularly provide information about their life quality over long periods. There’s the problem of making sure that comparable PROM data is collected consistently across health systems – so that it becomes a genuinely useful big data project. And there’s the problem of making sure that all that data, whether collected in trials or in everyday clinical practice, is actually used – in drug approvals, health technology assessments and treatment choices.
For Calvert, at Birmingham University, one of the main challenges to address is the multiplicity of PROM data capture systems being used to address different stakeholders’ needs. “We are currently working with patients, clinicians and other stakeholders to understand their needs, and are developing systems for efficient PROM data capture in the UK National Health Service,” she says.
To achieve consistency, the Centre for Patient Reported Outcomes Research is recommending that all clinical trials use its new international PROM protocol guidance, which was developed with international collaborators (SPIRIT-PRO). PROM data would more easily inform patient care if PROM reporting guidelines, such as CONSORT-PRO were used, according to Calvert.
The other big challenge is to make the act of capturing data as easy and effective for the patient as possible. “There’s a risk of over-burdening patients,” she says.
Completing quality of life questionnaires can often be time-consuming – and sometimes frustrating to patients if the questions don’t allow them to reflect what’s actually happening to them.
Asking the right questions
This is an issue that has been pre-occupying John Ware, Professor of Quantitative Health Sciences at the University of Massachusetts, who runs a research group aiming to standardise PROMs so that they can be used effectively to improve services. He is clear about the need for questionnaires that cover a broad range of domains and disease types, but which also allow the patient to zoom in and drill down into specific areas that are of concern to them at a particular time – and provide a “barometer” to their general wellbeing.
Ware’s team have developed ‘shortform’ digital questionnaires, reducing dozens of questions to less than ten by directing patients to respond only about the disease, symptoms and issues that matter to them at a particular time. He has demonstrated that, through the use of apps, gathering detailed actionable data without overburdening the patient is feasible.
The challenge now, he says, is to harmonise PROM tools on an international basis, so that all the data collected is comparable and useful. The harmonisation will involve incorporating already well-established generic PROMs with disease-specific PROMs, which provide the detailed data that cancer patients and clinicians really need. The measures produced by EORTC’s Quality of Life Group have already provided a good basis on which to build, says Ware. Its QLQ-C30 questionnaire to assess the quality of life of cancer patients has been validated in more than 100 languages and is used in more than 3,000 studies worldwide. It is supplemented with modules for specific types of cancer (see box overleaf).
“It’s the best generic tool, and what needs to happen is for it be more efficient and part of the move towards harmonisation,” says Ware. Roger Wilson agrees they are a good basis on which to build. “The EORTC QLQ-C30 and the range of tools developed alongside may be the best generic PROMs we have at the moment. The extensions cover different tumour types, and patients are engaged in their development.”
PROMS in cancer: the main players
The European Organisation for Research and Treatment of Cancer (EORTC)
- EORTC has long been involved in producing quality of life questionnaires for people with cancer.
- The most commonly used PROM in oncology is the QLQ-C30, which was launched by EORTC in 1988.
- QLQ-C30 is largely a generic quality of life tool, but has bolt-on modules for speci c cancers and their symptoms (see box p 60).
- QLQ-C30 was designed to be used mainly in the context of clinical trials.
- EORTC supports the routine use of PROMs in manuals and guidelines.
- EORTC’s SISAQOL project is developing an international set of data standards so that PROM data gathered in cancer research can be better compared and interpreted.
The Centre for Patient Reported Outcomes Research, Birmingham University, UK
- CPROR is researching how PROM use can be optimised in trials, applied research and routine practice.
- It is looking at the PROM guidance available to clinicians and study developers.
- It has been involved in the development of CONSORT-PRO and SPIRIT-PRO guidance – extensions of theCONSORT and SPIRIT guidance on methodological rigour and transparency in trials – to encourage high-quality reporting of PROMS.
- It works closely with patient partners.
John Ware Research Group, Boston, US
- The John Ware Research Group aims to standardise PROMs so that data from treatment outcome studies, individual patients, and populations can be compared, making information about outcomes more useful.
- Founder John Ware developed the SF-36 – an internationally used patient reported health survey.
- It recently developed new tools to: standardise PROM content and scoring across diseases; adapt to multiple chronic conditions in disease-specific measures; and integrate disease specific and generic measures.
Drug regulators
- The European Medicines Agency (EMA) and Food and Drug Administration (FDA) have issued guidance to researchers on the use of PROMs.
- The EMA has indicated that it is acceptable that quality of life and e cacy should be co-primary endpoints.
- The FDA has highlighted the importance of patients informing PROM content.
- Both the EMA and FDA are supporting EORTC’s SISAQOL initiative to standardise PROMs analysis.
Making PROMs count
The potential is clear to Efficace, Chair of the EORTC Quality of Life Group. He says that, in the past, lack of methodological rigour and statistical consistency in trials has been a major impediment to PROM information being used to guide clinical decision making. But things are changing.
“Performing research well, and presenting it well to the scientific community, is essential. But it is not yet sufficient to make a difference in the real world,” says Efficace. “The next step is to make sure that patient reported outcome data is considered by health policy makers and regulatory stakeholders, and actually influences future clinical decisions.
“I don’t have all the answers for how you make that happen, but what I can say is that, in Europe, it helps a great deal that the EMA has recently issued a document stating how patient reported outcome tools should be implemented in clinical trials. These kind of official endorsements from regulatory stakeholders help clarify a number of aspects that could guide future studies.”
He has shown that gathering detailed actionable data
without overburdening the patient is feasible
EORTC too has a leadership role to play – both in keeping its tools relevant as cancer treatments and their side effects change, and in standardising data and its analysis. In 2016, it launched its SISAQOL initiative (Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data). This aims to develop recommendations for standardising the analysis and interpretation of PROMs and quality of life data in cancer randomised trials.
Efficace believes the initiative is an important one: the challenge will be to make sure it is implemented, among all the other guidelines that researchers and clinicians are supposed to abide by. “We in EORTC should play a major role in raising awareness of the value of PROMs,” he says, “both with regulatory stakeholders and with the public in general. We are fully committed to this, and will continue to push it over the coming months.”
An example of PRO scales: the QLQ-C30
The QLQ-C30 patient questionnaire consists of both multi-item and single scales. These include:
- Functional scales: Physical, Role, Emotional, Social, Cognitive;
- Symptom scales: Fatigue, Nausea and vomiting, Pain;
- Global health status/QOL scale;
- Other items: Dyspnoea, Insomnia, Appetite loss, Constipation, Diarrhoea, Financial di culties.
PROMs development starts with patients
For Roger Wilson, change needs to go even deeper, and the perspective needs to change more completely towards the patient. Impressed as he is with validated tools such as EORTC’s, patients of all types – not just ‘professional advocates’ – need to be involved in revising and widening them. The work of updating and standardising PROMs needs to be truly multidisciplinary.
“The idea that you can have patient reported outcomes without patient provided inputs to inform methods and processes is irrational and probably unethical,” he says.
For tools used specifically within cancer, the focus also needs to shift away from what researchers need to something that is used and valuable for every patient and in every clinic. “One of the problems with existing tools is that they have been developed in the context of randomised controlled trials. And although there is a growing library of tools covering specific cancers, they tend to be tumour specific, with a treatment focus,” says Wilson.
“There are fewer tools available for use in specific situations, apart from palliative care,” he adds. He cites the example of amputation, which is associated not just with cancer – as in his case – but also diabetes, vascular disease, and motorcycle accidents.
“Seven years ago I was involved in a project with palliative care sarcoma patients at the Royal Marsden. We used QLQ-C30 and several other instruments – getting a mix of generic and specific tools was the best we could do, because there was no sarcoma specific tool. There still isn’t, although one is in development.”
Will that kind of detailed development happen more widely? What will it take for Roger Wilson’s 42 to be more than science fiction?
It is achievable, he insists. And, like Efficace, he believes the key is increasing awareness and changing mindsets. “The big issue is people thinking, ‘Why do we need to do that?’ So it’s going to require a lot of willpower – a lot of real energy to get the word out and accepted. Nationally, PROMs need high profile leaders or organisations to provide credibility.” Governments, he fears, are unlikely to impose anything from on high, or provide cash for blue sky projects.
John Ware shares his scepticism: “Personally, I’m tremendously disappointed that governments, which are spending huge proportions of GDP on the maintenance of human health, are not taking a lead on standardising its conceptualisation and measurement,” he says.
So it may be down to the cheerleaders. And maybe the galvanising role of Roger Wilson, a man who will be spreading the word about PROMs at cancer events across the world during 2018 and 2019, will in the end prove crucial.
“Patients – and not just ‘professional advocates’ –
need to be involved in revising existing tools”
“Across all the areas that people talk about for improving cancer survival – better diagnostic techniques, faster routes to diagnosis, new drugs and treatment techniques – lies the issue of quality of life,” he says. “All the buzz and hype is about drugs, particularly in advanced disease, and what’s friendly to the patient gets forgotten. It’s about redressing the balance.”
And by prioritising the patient experience, it also happens to be rather revolutionary? “Oh yes, I love that,” says Wilson. “I’m all for a bit of revolution.”