Two practitioners of integrative oncology make the case for using non-traditional therapies alongside mainstream care and for abandoning the term complementary and alternative medicine as unhelpful and misleading in this comprehensive article, which first appeared in Nature Reviews Clinical Oncology.
Imagine this scene: the oncologist concludes an outline of an adjuvant treatment plan to a patient who has recently been diagnosed with stage III breast cancer. The patient asks, “Should I be on an alkaline diet? I heard that alkalising the body kills cancer cells. I’ve also heard that sugar feeds cancer. Should I avoid sugar? How about graviola, a herb from the Amazon that is supposed to cure cancer? Can I get acupuncture during chemotherapy to reduce side effects?” This not-uncommon scenario brings to mind pressing questions. What are these therapies and remedies that are not traditionally part of Western mainstream medical care? How do we, as oncologists, respond to questions about nontraditional therapies?
Data on the use of adjunctive complementary therapies for symptom control is often confused by the use of the convenient acronym ‘CAM’ – complementary and alternative medicine – in publications that fail to distinguish between alternative and complementary modalities. The acronym is inherently imprecise. Some therapies, such as vitamins, are part of mainstream medical care when prescribed to patients with vitamin deficiencies or taken in appropriate amounts to maintain general health. However, vitamins are ‘alternatives’ when used in ‘megadoses’ as a treatment for cancer, sometimes in lieu of mainstream care. Similarly, ‘prayer for health’ might be a useful aid during mainstream cancer care in some regions of the world for some patients, but can be selected as a cancer ‘treatment’ in others. Thus, the terminology and its varying interpretations interfere with accurate reporting and hinder the accurate understanding of survey data.
The interest in therapies outside of mainstream oncology care is not limited geographically or among particular segments of the population. In countries in which modern medicine predominates, 40–50% of patients with cancer use CAM therapies outside the mainstream.[1–6] Among cancer survivors in the US, up to 40% used complementary or alternative therapies during the period following their treatment.[7] The 2007 National Health Interview Survey showed that four in ten adults (38.3% of adults; 83 million individuals) and one in nine children less than 18 years of age (11.8% of children; 8.5 million individuals) in the US used dietary supplements and various mind–body therapy techniques.[8,9]
Largely because the CAM terminology is an admixture of unrelated – often mutually contradictory – concepts, the term has become outdated and is no longer in common use. As accurately stated in a recent publication: “the term ‘integrative medicine’ is fast replacing that of complementary and alternative medicine, or ‘CAM.’”[10] Another publication sums up the terminology problem well: “this controversial term should be changed, since the words ‘complementary’ and ‘alternative’ have different meanings and should not be connected by ‘and’.”[11] Complementary therapies are those used to complement or use alongside conventional methods of therapy, whereas alternative methods refer to those that are used instead of known conventional therapies. Accordingly, the term ‘integrative therapies’ accurately describes the complementary treatments being used in medical settings alongside conventional practices. Centres for integrative medicine are being established in many academic medical centres.[11] Indeed, the term CAM is rarely applied in legitimate settings, and virtually every National Cancer Institute (NCI)-designated comprehensive cancer centre in the US has a programme or department using the term ‘integrative medicine’. In addition, the US Consortium of Academic Health Centers for Integrative Medicine has a membership of 55 esteemed academic medical centres with medical schools, all of which have integrative medicine programmes.
In this article, we summarise the data on helpful complementary therapies and their appropriate incorporation into cancer care (integrative oncology), discuss nonviable alternative therapies and examine the patient interest in these therapies. We also provide recommendations for how oncology professionals can manage these issues in an evidence-based, compassionate fashion that enhances trust and rapport, strengthens the physician–patient relationship and improves the quality of life for both the patient and their caregivers.
Complementary approaches
Mind–body therapies
Mind–body modalities focus on interactions between the brain, mind, body and behaviour with the intention of reducing symptoms and promoting health. Some of these therapies, such as meditation, relaxation techniques, hypnotherapy, yoga, T’ai Chi, music therapy and qigong have ancient roots; others, more recently developed, include the likes of guided imagery.[12] The common goal of mind–body therapies is to reduce the effects of anxiety, fear, phobia, anger, resentment, depression and pain on the patient while promoting a sense of emotional, physical and spiritual well-being. Mind–body therapies do not treat cancer per se.
Numerous clinical trials of variable quality have been conducted to assess the benefits of these techniques. For example, systematic reviews and meta-analyses have consistently shown that mind–body techniques do reduce anxiety and stress, improve sleep quality and overall quality of life, especially when used with other treatments (such as drugs).[13–17] Among such therapies, mindfulness-based stress reduction is the best studied – an approach that focuses on developing the patient’s objective ‘observer role’ for emotions, feelings and perceptions and creating a nonjudgmental ‘mindful state’ of conscious awareness.[18] Its meditative components of body scan, sitting meditation and mindful movement are taught over a period of weeks.[18] By contrast, yoga, T’ai Chi and qigong, which originated in Asia, are less well studied, despite being commonly used. They combine physical movement, postures and breath control with meditation. A few small trials (20–80 patients) have shown a reduction in anxiety, depression and distress as well as improved emotional well-being in patients with cancer who practice these techniques, as measured by standard validated instruments.[19–21]
Although mind–body therapies are generally safe, their effectiveness requires instructors skilled in conveying appropriate technique and regular practice by the patient. These helpful complementary modalities can be used as part of a multidisciplinary approach to patient care. Major research studies are underway to elucidate the mechanisms by which mental activity exerts control over physiological function,[22–24]
Acupuncture
Acupuncture, an ancient technique with great contemporary interest, involves the placement of special needles at certain body points (acupoints) a few millimetres to a few centimetres into the skin, which can be followed by manual manipulation or the application of heat or electric pulses to the needles.[25] Historically, acupuncture was thought to exert its effect by regulating the flow of energy (called chi or qi) along meridians in the body when inserted into these acupoints.[25] Although anatomical studies have shown that acupoints tend to be located over interstitial connective tissue planes,[26] current evidence does not conclusively support the claim that acupuncture points or meridians are electrically distinguishable.[27] However, substantial data from neuroscientific research suggest that the effects of acupuncture are mediated via modulation of nervous system activity.[28–31] Regulated as medical devices in the US, acupuncture needles are sterile, single-use, filiform, 32–36 gauge and 30–40 mm in length. A typical treatment session is provided by licensed or certified professionals and lasts 20–40 minutes.
Acupuncture is used to treat a wide variety of ailments, although its efficacy has been evaluated with rigorous scientific research methodology only in the past few decades. Clinical trials have shown that the treatment is safe and effective for several symptoms experienced by patients with cancer.[32] Indeed, a Cochrane review of 11 randomised controlled trials (RCTs) encompassing 1,247 patients – most using sham acupuncture as controls – concluded that acupuncture reduces chemotherapy-induced nausea and vomiting.[33] The majority of acupuncture trials have been conducted to determine the efficacy of acupuncture in reducing pain. Recent systematic reviews of RCTs support the analgesic effects of acupuncture for certain types of pain (for example, musculoskeletal pain, osteoarthritis and chronic headache).[34, 35] Furthermore, acupuncture has shown benefit in reducing radiation-induced xerostomia,36 but mixed results in reducing hot flushes experienced by women with breast cancer.[37–39] The technique is possibly effective in reducing lymphoedema in women with breast cancer who had axillary dissection.[40] Both a systematic review of 46 RCTs and a Cochrane review showed that acupuncture seems effective in treating insomnia, although larger, rigorously designed RCTs are warranted.[41] Acupuncture has also been shown to relieve anxiety in a diverse patient population.[42–44]
Acupuncture is generally safe when performed by qualified practitioners. After 760,000 treatments in 97,733 patients receiving acupuncture in Germany, only six cases of treatment-related serious adverse events were reported.45 The most common adverse effects (<5%) included minor bleeding or bruising and pain or unfamiliar sensations at the acupuncture sites. In patients with cancer, acupuncture should not be given to those with severe neutropenia or thrombocytopenia due to their higher risk of infection or bleeding, or at the site of primary or metastatic neoplasm.
Acupuncture is not an optimum first-line treatment for symptom relief. Rather, it can be considered when standard treatment is not satisfactory or not tolerated. In patients with severe chemotherapy-induced nausea, vomiting, pain, xerostomia or hot flushes in spite of optimal medical management, acupuncture can be included as part of a multimodal management plan. Although some insurance companies do cover acupuncture treatment provided by qualified therapists for certain indications, the cost-effectiveness of acupuncture remains to be determined.
Manipulative and body-based practices
Massage therapy and other manual techniques – such as Swedish massage, shiatsu, tui na, reflexology, Thai massage, Ayurvedic massage, lymphatic drainage and myofascial release – are provided by massage therapists, physical therapists and occupational therapists.[46] These practices, which evolved from various cultures, focus primarily on the musculoskeletal system and connective tissues. For example, Swedish massage, the most commonly practised massage therapy in the West, uses five styles of long, flowing strokes – effleurage (gliding), petrissage (kneading), tapotement (rhythmic tapping), friction and vibration/shaking – to manipulate soft tissues.
Most cancer-related clinical trials of massage therapy focus on Swedish massage and reflexology (foot massage using specified parts of the sole thought to relate to bodily organs or locations). Results have been summarised in two systematic reviews that incorporate 14 RCTs and 12 RCTs, respectively, with some overlap.[47,48] The control interventions used in these trials include standard of care, attention (where patients received interpersonal interactions but not massage therapy) or low-intensity bodywork, such as light touch. Although the reviewers indicate that the research methodology of most trials included in both reviews was poor – for example, small sample sizes or the lack of any attempt to control for nonspecific effects – the data do support massage therapy as an effective adjunct in cancer supportive care to reduce anxiety and pain.[47,48]
Massage therapy in patients with cancer must be provided by certified massage therapists who are also trained in working with patients with cancer, to minimise risk of injury. For example, only light-touch massage should be provided to frail patients. Strong pressure should be avoided in areas harbouring tumours or metastases, or to patients with bleeding tendencies. Cases of serious adverse events – including cerebrovascular accidents, ureteral stent displacement, haematoma, nerve damage and posterior interosseous syndrome – have been reported, usually as a result of exotic types of massage (such as application of very strong pressure not appropriate for the anatomical location) or massage delivered by laypeople.[49,50] When delivered appropriately, massage therapy is a valuable, soothing complementary therapy that aids symptom control in patients with cancer and on which many patients rely.
Alternative therapies
Patients might seek alternative therapies for a variety of reasons, including frustration with a lack of improvement using mainstream treatments. In the past, cancer was considered a dire disease with few effective treatments; accordingly, patients sought more-effective, gentler treatments – real or imaginary.[51]
Today, the primary danger of alternative therapies is that patients delay or forego altogether effective cancer treatment. For example, instead of undergoing surgical resection of an early-stage breast cancer, a patient might opt for an alternative ‘natural therapy’. By the time it becomes apparent to the patient that the therapy has not controlled the growth of her cancer, it has metastasised, rendering it incurable. Another risk to patients is that most alternative therapies are very costly. And as these are rarely covered by insurance schemes, patients must pay out of pocket for them, often depleting their resources with the false hope that they are receiving effective therapy. The subsequent financial havoc creates tremendous distress for the patient and family. A third risk is that these therapies make false promises to desperate patients – results that cannot be delivered, representing an act of deception and betrayal. As clinicians, we have a moral obligation to dissuade patients from these useless therapies.
Miraculous cancer cures
Proponents of alternative therapies claim to produce ‘amazing’ results in patients with cancer who have not responded to conventional therapy. The treatment can be as simple as a single product from an exotic source or derived from a ‘breakthrough discovery’ decades ago yet ‘suppressed’ by mainstream medicine thereafter. For example, amygdalin (also known as laetrile) is an extract from bitter apricot seeds that is not – despite its other moniker of vitamin B17 – a vitamin. Although clinical studies have shown a lack of efficacy[52,53] and a risk of cyanide toxicity,[54] some patients continue to seek and use it. In our own recent experience, one patient proudly displayed her vitamin B17 pill bottle during a consultation and claimed that someone told her it cured his cancer after he had been told he had only months to live. We are certain other oncologists have been faced with similar situations.
Another touted miracle cure is caesium therapy, in which patients ingest caesium chloride (CsCl) to alkalinise the body. Proponents claim CsCl will kill the cancer cells because cancer cells “perish in an alkaline, high-pH, environment.”[55] Unfortunately, ingestion of CsCl can lead to torsade de pointes, a potentially lethal cardiac arrhythmia.[56,57] This alternative treatment can also include an elaborate regimen of special diets, detoxification techniques and large doses of natural products. Furthermore, these therapies rely heavily on testimonials of purported users of the products; the promotional materials are often laden with specious scientific jargon that can appeal to laypersons, but their misleading nature is obvious to anyone versed in cancer biology. Other examples include ‘oxygen therapy’ (ingestion or injection of substance containing hydrogen peroxide or ozone) and variations of bioelectromagnetism (subjecting the body to electromagnetic field generated by a device), as are various ‘energy therapies’.[58]
The parties that stand to profit from these products use various tactics to circumvent laws and regulations. They often use carefully worded statements or testimonials to create the impression that the products can cure cancer without literally saying so. Or, they disassociate themselves from the promoters by engaging in multilevel marketing or ‘guerrilla marketing’ schemes. Although the FDA (US regulatory body) has investigated numerous unsubstantiated claims and the Federal Communications Commission (FCC) investigates such false advertisements, the resources required to gather evidence and initiate legal actions are such that they can only prosecute a small number of violators. Physicians should educate their patients about why these therapies should be avoided.
Anticancer diets
A near-universal patient question concerns diet. Often patients are not satisfied with the usual dietary advice offered by dieticians, and seek ‘anticancer’ diets – an approach that has spawned its own category of self-help books.
Alkaline diets
One example that is frequently cited in this category is the so-called alkaline or pH diet. This diet is similar to the concept behind caesium therapy, that acidity promotes cancer and cancer cells cannot survive in an alkaline environment. By drinking ‘alkaline water’, distributed from an expensive device hooked up to a faucet [tap], and eating ‘alkaline foods’, which happen to be mainly fresh vegetables, fruits, legumes and nuts, one can ward off cancer, arthritis, obesity and other diseases. These claims disregard the fact that the body maintains a tight pH range and eliminates excess acid or alkaline to preserve pH balance. Treated water has little buffering capacity, therefore, drinking so-called alkaline water will not significantly affect blood pH levels. Similarly, glorifying alkaline foods simply translates to eating food that is healthy, which provides essential nutrients and not an alkaline environment toxic to cancer cells.
Other anticancer diets
Many other anticancer diets with little scientific basis circulate among patients, including the Budwig diet, the Gerson diet, the raw food diet and many more.[58] In addition, ‘detox’ or ‘mono’ diets (such as those relying mainly on vegetable and fruit juice) can restrict or preclude important food categories that are necessary for a full range of nutrition.
A high-fat, low-carbohydrate ketogenic diet is another popular subject of inquiry. Animal studies suggest that ketogenic diets induce excessive oxidative stress and might enhance the therapeutic effects of radiotherapy.[59] Clinical trials are underway to evaluate their benefits and risks.[60–62]
When responding to diet-related inquiries, oncologists might find it helpful to point to the ‘kernels’ of truth in the marketing materials for these programmes. The basic requirements for optimal health are to consume a variety of wholesome fresh foods and to reduce the intake of processed food. None of the radical anticancer diets that employ restrictive regimens have been shown to significantly improve survival. As such, patients adhering to these schemes run the risk of malnutrition. Optimal caloric and nutrient intake is very important for patients to be able to withstand their cancer therapies. Counselling patients who ask about these diets also provides a good opportunity to put dietary advice in the context of an overall healthy lifestyle, which also includes regular exercise.[63] The potential for physical activity to improve outcomes,[64,65] including benefits to patients receiving palliative care,[66,67] should be noted. Many leading cancer facilities have exercise programmes tailored to the needs of patients with cancer, with experienced fitness instructors who routinely work with patients across a broad range of abilities and disabilities.
Sugar and cancer
The notion that sugar ‘feeds’ cancer is frequently cited by concerned patients. Although not entirely without merit – glucose metabolism is an active area of research in anticancer drug development[68] – it is often exaggerated in public perception. Some patients become paranoid about all sugar-containing food, regardless of the amount. Such anxiety by itself is detrimental to the quality of life of patients and should be avoided. Instead, patients should be advised to keep things in perspective: no definitive data have shown that sugar promotes cancer growth. However, excessive intake of refined sugar is unhealthy for many reasons, especially for its association with metabolic syndrome, and should accordingly be minimised. A small amount of refined sugar is not harmful. To meet caloric requirements, patients should consume complex, unrefined carbohydrates and unsaturated fat (unless they have a digestive tract condition that precludes those foods) and avoid large amounts of foods with added sugar.[69]
Natural product dietary supplements
The use of supplements is among the most frequently questioned subjects by patients. Over-the-counter dietary supplements available to patients with cancer include vitamins and trace-element formulations that have well-defined constituents. Additionally, botanical extracts and herbal products that often contain complex compositions of many compounds, some of which are unidentified, are also available. Indeed, botanicals, fungi and marine organisms (such as sea sponges) are a rich source of therapeutic compounds that are used in cancer therapy; chemotherapeutic agents derived from natural sources – so-called natural products in chemical jargon – include the taxanes (paclitaxel is isolated from the Pacific Yew tree), camptothecin (isolated from of C. acuminata, the Chinese ‘happy tree’) and its analogues, vinca alkaloids (isolated from the periwinkle C. roseus) and numerous microbial compounds.[70] However, decades of research are needed to ascertain the clinical safety and efficacy of compounds derived from natural sources in clinical studies. Natural products available as dietary supplements are not viable cancer treatments until their efficacy has been established in such studies.
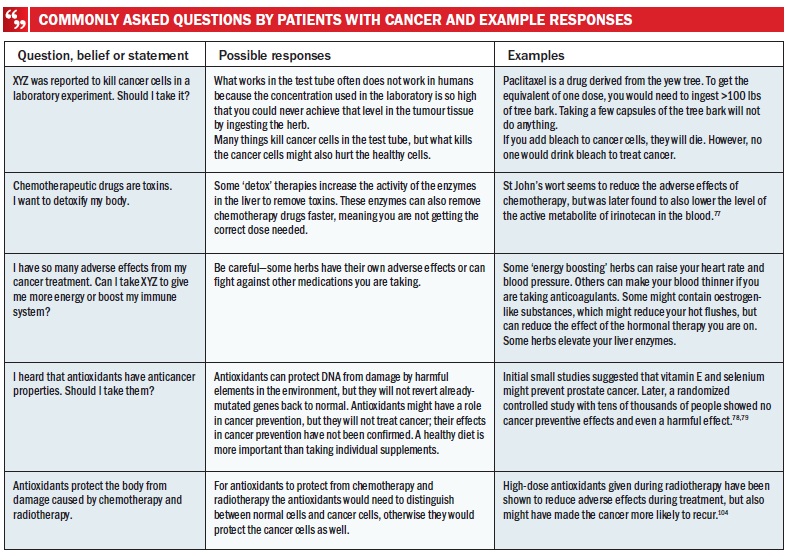
Patients with cancer often ask about natural products that have been shown to have activity against cancer in animal or in vitro studies, but unconfirmed in clinical trials. Many are readily available as dietary supplements, and patients can use them on their own, often without informing their physicians. In addition, patients might pursue the use of natural products marketed with ‘buzz-words’ such as antioxidant, immune booster or detox. A comprehensive list of such supplements has been reported.[71] Patients view these products as helpful during cancer treatment because of claims that the products protect ‘good’ cells from damage, restore suppressed immune function or remove toxins ‘left behind’ by cancer treatment. Some of these agents might hold promise in cancer prevention or treatment; for example, early-phase clinical trials of polyphenols extracted from green tea have demonstrated benefit in the treatment of chronic lymphocytic leukaemia (CLL)[72] and in the chemoprevention of breast cancer.[73] Similarly, docosahexanoic acid (DHA, an Ω3-fatty acid) has demonstrated positive results in breast cancer prevention[74] and treatment,[75] as has curcumin in slowing progression from monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma.[76] Nonetheless, the majority of the available supplements has not shown a reasonable possibility of meaningful clinical benefits when ingested orally. Many also carry the risk of interacting with prescription medicines or have their own detrimental effects.[77–80] For example, several herbs possess oestrogen-like activity, the intake of which is not advisable for women with oestrogen-receptor-positive cancers.[81] Other supplements can alter drug metabolism, such as St John’s wort, leading to serum drug levels higher or lower than intended.[82] Misconceptions held by patients after reading news reports or marketing materials need to be invalidated in a language patients can understand (see Table below).
 Patient characteristics
Patient characteristics
Surveys indicate that patients with cancer who use complementary or alternative approaches tend to be female as well as younger, better educated and more affluent than those who do not, representing a health-conscious segment of the population that is proactive in its healthcare, that seeks health information and that has the means to pay for services not typically covered by insurance or public health schemes.[1–6] Given the increased sophistication of patients and physicians in recent years, patients and their oncologists increasingly pursue discussions of integrative oncology, alert to the fact that incorporating complementary (adjunctive) therapies into mainstream cancer treatment can decrease symptoms and improve overall quality of life. Furthermore, such discussions facilitate patients in having an active role in their care.[83]
Patients acquire information about complementary approaches primarily from friends (65%), family (48%) and the media (21%),[1,84] with additional information – as well as misinformation – being delivered via the internet and social networks.[85] The general interest in complementary modalities has increased in recent decades because of increasing supportive data on the value of complementary therapies, as well as growing professional and patient acceptance of the modalities used. Additionally, the emphasis on wellness and survivorship, which incorporates managing long-term adverse effects from cancer treatment and reducing risk of cancer recurrence, has enhanced general interest in complementary modalities.
Impediments to communication
Optimal cancer care demands dealing with issues that are important to patients, including those that are likely to be detrimental if left unaddressed. Proper dialogue about the use and application of therapies is important. The majority of patients want to discuss the topic with their oncologists given the opportunity, yet nondisclosure remains a problem, in part because the opportunity fails to arise.[86] Additionally, patients have also reported being fearful of physician disapproval or disinterest in what they do outside of conventional treatment, or assume that such information is not important or relevant to their cancer treatment.[87,88] By contrast, physicians believe that patient nondisclosure is attributable to patient fears of physician disapproval or lack of understanding.[89] As the prevalence of complementary therapy use is high, initiating a discussion provides an excellent opportunity for the physician to demonstrate compassion, understanding and humanity, in addition to providing high-quality care based on scientific data.
Dilemmas for clinicians
Although most patients use natural products (supplements) hoping to reduce the adverse effects of conventional treatment, support the body through cancer treatment or prevent cancer recurrence, other patients with few or no effective treatment options might want to try anything. This desperation can lead the patient to use natural products that have shown some possible anticancer activity in early preliminary studies. Although the chances of efficacy might be extremely remote, these patients might come to the clinic with information about the benefits of various elaborate, so-called anticancer regimens.
One example is the Bill Peeples cocktail, which is often asked about by patients with advanced-stage sarcoma.[90] This product contains more than a dozen dietary supplemental ingredients that the manufacturer claims have antiangiogenic or antioxidant properties based on laboratory studies, plus a few prescription medicines used off-label. Such a scenario presents a dilemma for the oncologist faced with a patient for whom there is no effective treatment or appropriate clinical trial. How do we provide compassionate care while safeguarding our patient’s best interests?
We believe that the answer lies in meeting patients where they are. We can affirm their perseverance not to give up, and tolerate their use of agents that are generally safe and have shown some preliminary evidence of anticancer activity. At the same time, we also need to help the patient work towards accepting whatever outcome he or she will eventually face, despite their best efforts and those of the treating physicians and caregivers. Palliative care often begins too late in clinical practice.[91] Instead, patients’ options, goals and preferences should be assessed early in the course of cancer treatment. Personalised care of patients with advanced-stage cancer should be tailored to the diverse physical, psychological, social and spiritual consequences of cancer for the individual.[92] Using complementary or alternative therapies might help patients feel content that they have explored every possible option, help them to accept the futility of further treatment and facilitate closure. Accordingly, the treating oncologist must take a compassionate approach in accepting these decisions by the patient. Similarly, an effort must be made to minimise the physical, emotional and financial burdens experienced by the patient, discuss and closely monitor adverse reactions and prepare the patient and family for end-of-life issues.
Integrative oncology programmes
Combining helpful complementary therapies with mainstream cancer care to reduce symptoms and improve quality of life constitutes the practice of integrative oncology. Many, if not most, cancer centres have established integrative oncology departments or programmes to provide complementary therapies and to counsel patients about potentially problematic dietary supplements and alternative therapies. Counselling by trained and experienced physicians should include guiding patients away from potentially harmful therapies and addressing their underlying psychosocial or cultural needs.[93,94] Referring patients to qualified specialists, therapists, counsellors or instructors connects these individuals to appropriate sources of facts and sound advice. These qualified personnel can serve as valuable resources for future questions, to provide support for patients’ efforts, and to divert energy away from useless and potentially harmful or expensive approaches. Furthermore, as patients gain information about additional symptom management techniques, they experience positive interactions with their physicians, improve self-care skills and enhance their physical, emotional and overall well-being.[95,96]
A structured approach to discussing the use of complementary or alternative medicine with patients in general was described in 1997 and updated in 2002.[97,98] Although written for use in the primary care or internal medicine settings, this approach might be helpful in the oncology setting as well. A similar report focused on discussing complementary or alternative medicine with patients in the oncology care setting.[93] In 2010, a set of comprehensive communication guidelines was proposed on the basis of a systematic literature review of methods for the discussion of complementary modalities for oncologists.[94] Together, these works provided a framework for counselling patients with cancer on complementary or alternative therapies.
At Memorial Sloan–Kettering Cancer Center, each new patient receives an information packet that reminds them to discuss any self-prescribed supplements or medications with their physicians. All patients are asked at each visit to disclose any herbs and other dietary supplements they are taking. Patients who raise questions about complementary or alternative therapies that require discussion – and those who are on supplements at risk of interacting with prescription medicines – are referred to the Integrative Medicine Service for comprehensive counselling. During the counselling (see Figure below), physicians well versed in both oncology and integrative medicine make a comprehensive assessment of the patient’s needs and address the issues from both the overall cancer care perspective and the patient-specific perspective.
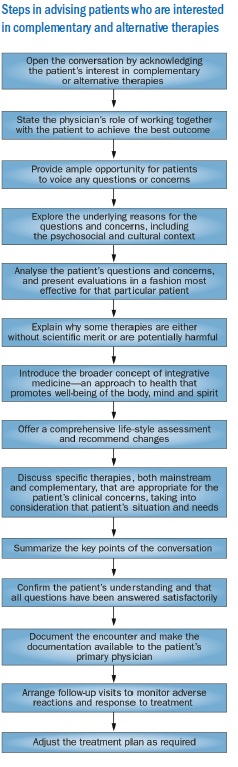 Practice models of integrative oncology vary according to the patient demographics and the societal environment of the medical facility. The integration is not always easy and can be hampered by a lack of awareness or perceived importance by oncologists, a lack of properly trained physicians knowledgeable in both cancer medicine and complementary therapies, a lack of trust between physicians and complementary therapies practitioners who are not medical doctors or insufficient funds. An investigation of six integrative oncology programmes across four continents identified several essential elements for a successful programme: location within the oncology department area, oncologist referral to consultation with integrative oncologist, sufficient time for integrative oncologist–oncologist communication, integrative practice that is evidence based, professional complementary medicine practitioners and coverage of the cost for the integrative oncology service.[99]
Practice models of integrative oncology vary according to the patient demographics and the societal environment of the medical facility. The integration is not always easy and can be hampered by a lack of awareness or perceived importance by oncologists, a lack of properly trained physicians knowledgeable in both cancer medicine and complementary therapies, a lack of trust between physicians and complementary therapies practitioners who are not medical doctors or insufficient funds. An investigation of six integrative oncology programmes across four continents identified several essential elements for a successful programme: location within the oncology department area, oncologist referral to consultation with integrative oncologist, sufficient time for integrative oncologist–oncologist communication, integrative practice that is evidence based, professional complementary medicine practitioners and coverage of the cost for the integrative oncology service.[99]
For busy oncologists, staying abreast of new complementary medicine research results and of the ever-expanding world of alternatives to mainstream cancer treatment is difficult. However, many excellent continuing education materials are available from reputable sources. In addition, the knowledge and expertise available from integrative oncology colleagues can be extremely helpful, especially those dual-trained in mainstream oncology and integrative medicine. National-level and international-level efforts can provide helpful information to practising oncologists. A multidisciplinary nonprofit organisation – the Society for Integrative Oncology – was formed by clinicians, researchers and patient advocates to provide a platform for the advancement of evidence-based, comprehensive, integrative healthcare to improve the lives of people affected by cancer.[100] Using the standard methodology for development of a practice guideline, which consists of systematic review of current literature and multiple rounds of peer reviews, the group evaluated the strength of the evidence for common complementary therapies, as well as any potential risks or burdens.
The resulting recommendations were graded, peer-reviewed and adapted by the American College of Chest Physicians[101,102] and the Society for Integrative Oncology.[103] These guidelines represent an initial effort in giving clinicians who might not be familiar with complementary therapies evidence-based assessments of the therapies, and when and how to incorporate them into the care of patients with cancer. With time, these national and international efforts in raising the awareness of integrative oncology and its application in clinical care would improve the overall care of cancer patients.
Conclusions
Complementary or alternative medicine are topics that patients with cancer are highly interested in and also find quite confusing. Safe and beneficial complementary therapies should be integrated into regular cancer care to improve patient quality of life and outcome. However, patients should be steered away from alternative cancer therapies that are risky and do not have clinical value. Integrative oncology combines complementary therapies with mainstream care, trying to optimise the patient’s physical, psychological and spiritual well-being, taking into consideration the individual’s values and priorities in life. A robust integrative oncology programme should be part of any high-quality cancer care institution, as is the case for virtually all NCI-designated comprehensive cancer centres. By understanding and addressing issues our patients feel are important, compassionate care can be tailored to each patient, and oncology will reach the noble goal of treating each patient as a person with cancer, rather than treating only the cancer in a patient.
This article was first published in Nature Reviews Clinical Oncology vol. 10 no.11, and is published with permission. © 2013 Nature Publishing Group. doi:10.1038/nrclinonc.2013.125
Author affiliations
Gary Deng and Barrie Cassileth, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, New York
References
1. Amin, M. et al. Complementary medicine use in patients with head and neck cancer in Ireland. Eur. Arch. Otorhinolaryngol. 267, 1291–1297 (2010).
2. Navo, M. A. et al. An assessment of the utilization of complementary and alternative medication in women with gynecologic or breast malignancies. J. Clin. Oncol. 22, 671–677 (2004).
3. Vapiwala, N., Mick, R., Hampshire, M. K., Metz, J. M. & DeNittis, A. S. Patient initiation of complementary and alternative medical therapies (CAM) following cancer diagnosis. Cancer J. 12, 467–474 (2006).
4. Molassiotis, A. et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann. Oncol. 16, 655–663 (2005).
5. Hyodo, I. et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J. Clin. Oncol. 23, 2645–2654 (2005).
6. Moran, M. S. et al. A prospective, multicenter study of complementary/alternative medicine (CAM) utilization during definitive radiation for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 85, 40–46 (2013).
7. Gansler, T., Kaw, C., Crammer, C. & Smith, T. A population-based study of prevalence of complementary methods use by cancer survivors: a report from the American Cancer Society’s studies of cancer survivors. Cancer 113, 1048–1057 (2008).
8. Nahin, R. L., Barnes, P. M., Stussman, B. J. & Bloom, B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat. Report, 1–14 (2009).
9. Barnes, P. M., Bloom, B. & Nahin, R. L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat. Report, 1–23 (2008).
10. Barton, D. Integrative medicine: not just garnish. ONCOLOGY Nurse Ed. 26 (2012).
11. Rosenthal, D. S. & Doherty-Gilman, A. M. Integrative medicine and cancer care. Virtual Mentor 13, 379–383 (2011).
12. Elkins, G., Fisher, W. & Johnson, A. Mind–body therapies in integrative oncology. Curr. Treat. Options Oncol. 11, 128–140 (2010).
13. Shennan, C., Payne, S. & Fenlon, D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychooncology 20, 681–697 (2011).
14. Ledesma, D. & Kumano, H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psychooncology 18, 571–579 (2009).
15. Cramer, H., Lauche, R., Paul, A. & Dobos, G. Mindfulness-based stress reduction for breast cancer-a systematic review and meta-analysis. Curr. Oncol. 19, e343–e352 (2012).
16. Zainal, N. Z., Booth, S. & Huppert, F. A. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology 22, 1457–1465 (2013).
17. Piet, J., Würtzen, H. & Zachariae, R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 80, 1007–1020 (2012).
18. Fjorback, L. O., Arendt, M., Ornbøl, E., Fink, P. & Walach, H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatr. Scand. 124, 102–119 (2011).
19. Lin, K. Y., Hu, Y. T., Chang, K. J., Lin, H. F. & Tsauo, J. Y. Effects of yoga on psychological health, quality of life, and physical health of patients with cancer: a meta-analysis. Evid. Based Complement. Alternat. Med. 2011, 659876 (2011).
20. Smith, K. B. & Pukall, C. F. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology 18, 465–475 (2009).
21. Chan, C. L. et al. A systematic review of the effectiveness of qigong exercise in supportive cancer care. Support. Care Cancer 20, 1121–1133 (2012).
22. Bhasin, M. K. et al. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS ONE 8, e62817 (2013).
23. Saatcioglu, F. Regulation of gene expression by yoga, meditation and related practices: a review of recent studies. Asian J. Psychiatr. 6, 74–77 (2013).
24. Davidson, R. J. & McEwen, B. S. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat. Neurosci. 15, 689–695 (2012).
25. Kaptchuk, T. J. Acupuncture: theory, efficacy, and practice. Ann. Intern. Med. 136, 374–383 (2002).
26. Langevin, H. M. & Yandow, J. A. Relationship of acupuncture points and meridians to connective tissue planes. Anat. Rec. 269, 257–265 (2002).
27. Ahn, A. C. et al. Electrical properties of acupuncture points and meridians: a systematic review. Bioelectromagnetics 29, 245–256 (2008).
28. Huang, W. et al. Characterizing acupuncture stimuli using brain imaging with FMRI—a systematic review and meta-analysis of the literature. PLoS ONE 7, e32960 (2012).
29. Han, J. S. Acupuncture analgesia: areas of consensus and controversy. Pain 152 (Suppl. 3), S41–S48 (2011).
30. Moffet, H. H. How might acupuncture work? A systematic review of physiologic rationales from clinical trials. BMC Complement. Altern. Med. 6, 25 (2006).
31. Stone, J. A. & Johnstone, P. A. Mechanisms of action for acupuncture in the oncology setting. Curr. Treat. Options Oncol. 11, 118–127 (2010).
32. Garcia, M. K. et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J. Clin. Oncol. 31, 952–960 (2013).
33. Ezzo, J. M. et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Systematic Reviews, Issue 2. Art. No.: CD002285 http://dx.doi.org/10.1002/14651858.CD002285.pub2.
34. Vickers, A. J. et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch. Intern. Med. 172, 1444–1453 (2012).
35. Lee, M. S. & Ernst, E. Acupuncture for pain: an overview of Cochrane reviews. Chin. J. Integr. Med. 17, 187–189 (2011).
36. Pfister, D. G. et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. J. Clin. Oncol. 28, 2565–2570 (2010).
37. Deng, G. et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J. Clin. Oncol. 25, 5584–5590 (2007).
38. Carpenter, J. S. & Neal, J. G. Other complementary and alternative medicine modalities: acupuncture, magnets, reflexology, and homeopathy. Am. J. Med. 118 (Suppl. 12B), 109–117 (2005).
39. Lee, M. S., Shin, B. C. & Ernst, E. Acupuncture for treating menopausal hot flushes: a systematic review. Climacteric 12, 16–25 (2009).
40. Cassileth, B. R. et al. Acupuncture in the treatment of upper-limb lymphedema: results of a pilot study. Cancer 119, 2455–2461 (2013).
41. Cao, H., Pan, X., Li, H. & Liu, J. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J. Altern. Complement. Med. 15, 1171–1186 (2009).
42. Pilkington, K., Kirkwood, G., Rampes, H., Cummings, M. & Richardson, J. Acupuncture for anxiety and anxiety disorders—a systematic literature review. Acupunct. Med. 25, 1–10 (2007).
43. Wang, S. M. & Kain, Z. N. Auricular acupuncture: a potential treatment for anxiety. Anesth. Analg. 92, 548–553 (2001).
44. Eich, H., Agelink, M. W., Lehmann, E., Lemmer, W. & Klieser, E. Acupuncture in patients with minor depressive episodes and generalized anxiety. Results of an experimental study [German]. Fortschr. Neurol. Psychiatr. 68, 137–144 (2000).
45. Melchart, D. et al. Prospective investigation of adverse effects of acupuncture in 97,733 patients. Arch. Intern. Med. 164, 104–105 (2004).
46. Fritz, S. Mosby’s Fundamentals of Therapeutic Massage, 5e (Mosby, 2012).
47. Ernst, E. Massage therapy for cancer palliation and supportive care: a systematic review of randomised clinical trials. Support. Care Cancer 17, 333–337 (2009).
48. Wilkinson, S., Barnes, K. & Storey, L. Massage for symptom relief in patients with cancer: systematic review. J. Adv. Nurs. 63, 430–439 (2008).
49. Ernst, E. The safety of massage therapy. Rheumatology (Oxford) 42, 1101–1106 (2003).
50. Corbin, L. Safety and efficacy of massage therapy for patients with cancer. Cancer Control 12, 158–164 (2005).
51. Cassileth, B. R. Sounding boards. After laetrile, what? N. Engl. J. Med. 306, 1482–1484 (1982).
52. Milazzo, S., Ernst, E., Lejeune, S., Boehm, K. & Horneber, M. Laetrile treatment for cancer. Cochrane Database Systematic Reviews, Issue 11. Art. No.: CD005476 http://dx.doi.org/10.1002/14651858.CD005476.pub3.
53. Milazzo, S., Lejeune, S. & Ernst, E. Laetrile for cancer: a systematic review of the clinical evidence. Support. Care Cancer 15, 583–595 (2007).
54. Kalyanaraman, U. P., Kalyanaraman, K., Cullinan, S. A. & McLean, J. M. Neuromyopathy of cyanide intoxication due to “laetrile” (amygdalin). A clinicopathologic study. Cancer 51, 2126–2133 (1983).
55. Cesium carbonate & cesium chloride kills cancer cells. The Natural Cures Website They Don’t Want You to Know About [online] (2012) http://www.angelfire.com/az/sthurston/cesiumcarbonateforcancer.html.
56. Wiens, M. et al. Cesium chloride-induced torsades de pointes. Can. J. Cardiol. 25, e329–e331 (2009).
57. Pinter, A., Dorian, P. & Newman, D. Cesium-induced torsades de pointes. N. Engl. J. Med. 346, 383–384 (2002).
58. Cassileth, B. The Complete Guide to Complementary Therapies in Cancer Care: Essential Information for Patients, Survivors and Health Professionals (World Scientific Publishing Company, 2011).
59. Abdelwahab, M. G. et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE 7, e36197 (2012).
60. US National Library of Medicine. ClinicalTrials.gov [online], (2012) http://www.clinicaltrials.gov/ct2/show/NCT01716468.
61. US National Library of Medicine. ClinicalTrials.gov [online], (2012) http://www.clinicaltrials.gov/ct2/show/NCT01419587.
62. US National Library of Medicine. ClinicalTrials.gov [online], (2012) http://www.clinicaltrials.gov/ct2/show/NCT01535911.
63. Demark-Wahnefried, W., Rock, C. L., Patrick, K. & Byers, T. Lifestyle interventions to reduce cancer risk and improve outcomes. Am. Fam. Physician 77, 1573–1578 (2008).
64. Holick, C. N. et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol. Biomarkers Prev. 17, 379–386 (2008).
65. Holmes, M. D., Chen, W. Y., Feskanich, D., Kroenke, C. H. & Colditz, G. A. Physical activity and survival after breast cancer diagnosis. JAMA 293, 2479–2486 (2005).
66. Lin, M. R., Hwang, H. F., Wang, Y. W., Chang, S. H. & Wolf, S. L. Community-based tai chi and its effect on injurious falls, balance, gait, and fear of falling in older people. Phys. Ther. 86, 1189–1201 (2006).
67. Faber, M. J., Bosscher, R. J., Chin A., Paw, M. J. & van Wieringen, P. C. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch. Phys. Med. Rehabil. 87, 885–896 (2006).
68. Kaelin, W. G. Jr & Thompson, C. B. Q&A: Cancer: clues from cell metabolism. Nature 465, 562–564 (2010).
69. Doyle, C. et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J. Clin. 56, 323–353 (2006).
70. Mann, J. Natural products in cancer chemotherapy: past, present and future. Nat. Rev. Cancer 2, 143–148 (2002).
71. Memorial Sloan–Kettering Cancer Center. Integrative Medicine Search About Herbs [online], (2013) http://www.mskcc.org/cancer-care/integrative-medicine/about-herbs.
72. Shanafelt, T. D. et al. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer 119, 363–370 (2013).
73. Crew, K. D. et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev. Res. (Phila.) 5, 1144–1154 (2012).
74. Yee, L. D. et al. ω3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am. J. Clin. Nutr. 91, 1185–1194 (2010).
75. Bougnoux, P. et al. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br. J. Cancer 101, 1978–1985 (2009).
76. Golombick, T., Diamond, T. H., Manoharan, A. & Ramakrishna, R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled cross-over 4 g study and an open-label 8g extension study. Am. J. Hematol. 87, 455–460 (2012).
77. Mathijssen, R. H., Verweij, J., de Bruijn, P., Loos, W. J. & Sparreboom, A. Effects of St John’s wort on irinotecan metabolism. J. Natl Cancer Inst. 94, 1247–1249 (2002).
78. Klein, E. A. et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306, 1549–1556 (2011).
79. Lippman, S. M. et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 301, 39–51 (2009).
80. Bairati, I. et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J. Clin. Oncol. 23, 5805–5813 (2005).
81. Deng, G., Davatgarzadeh, A., Yeung, S. & Cassileth, B. Phytoestrogens: science, evidence, and advice for breast cancer patients. J. Soc. Integr. Oncol. 8, 20–30 (2010).
82. Izzo, A. A. & Ernst, E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69, 1777–1798 (2009).
83. Verhoef, M. J., Rose, M. S., White, M. & Balneaves, L. G. Declining conventional cancer treatment and using complementary and alternative medicine: a problem or a challenge? Curr. Oncol. 15 (Suppl. 2), s101–s106 (2008).
84. Verhoef, M. J., Balneaves, L. G., Boon, H. S. & Vroegindewey, A. Reasons for and characteristics associated with complementary and alternative medicine use among adult cancer patients: a systematic review. Integr. Cancer Ther. 4, 274–286 (2005).
85. Page, S. A., Mannion, C., Bell, L. H. & Verhoef, M. J. CAM information online: an audit of Internet information on the “Bill Henderson Protocol”. Complement. Ther. Med. 18, 206–214 (2010).
86. Saxe, G. A. et al. Disclosure to physicians of CAM use by breast cancer patients: findings from the Women’s Healthy Eating and Living Study. Integr. Cancer Ther. 7, 122–129 (2008).
87. Tasaki, K., Maskarinec, G., Shumay, D. M., Tatsumura, Y. & Kakai, H. Communication between physicians and cancer patients about complementary and alternative medicine: exploring patients’ perspectives. Psychooncology 11, 212–220 (2002).
88. Robinson, A. & McGrail, M. R. Disclosure of CAM use to medical practitioners: a review of qualitative and quantitative studies. Complement. Ther. Med. 12, 90–98 (2004).
89. Richardson, M. A., Mâsse, L. C., Nanny, K. & Sanders, C. Discrepant views of oncologists and cancer patients on complementary/alternative medicine. Support. Care Cancer 12, 797–804 (2004).
90. Amschwand Sarcoma Cancer Foundation [online], (2013) http://www.sarcomacancer.org/.
91. Temel, J. S. et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 363, 733–742 (2010).
92. Peppercorn, J. M. et al. American Society of Clinical Oncology statement: toward individualized care for patients with advanced cancer. J. Clin. Oncol. 29, 755–760 (2011).
93. Cohen, L., Cohen, M. H., Kirkwood, C. & Russell, N. C. Discussing complementary therapies in an oncology setting. J. Soc. Integr. Oncol. 5, 18–24 (2007).
94. Schofield, P., Diggens, J., Charleson, C., Marigliani, R. & Jefford, M. Effectively discussing complementary and alternative medicine in a conventional oncology setting: communication recommendations for clinicians. Patient Educ. Couns. 79, 143–151 (2010).
95. Koithan, M., Bell, I. R., Caspi, O., Ferro, L. & Brown, V. Patients’ experiences and perceptions of a consultative model integrative medicine clinic: a qualitative study. Integr. Cancer Ther. 6, 174–184 (2007).
96. Verhoef, M. J., Mulkins, A. & Boon, H. Integrative health care: how can we determine whether patients benefit? J. Altern. Complement. Med. 11 (Suppl. 1), S57–S65 (2005).
97. Eisenberg, D. M. Advising patients who seek alternative medical therapies. Ann. Intern. Med. 127, 61–69 (1997).
98. Weiger, W. A. et al. Advising patients who seek complementary and alternative medical therapies for cancer. Ann. Intern. Med. 137, 889–903 (2002).
99. Ben-Arye, E. et al. Integrating complementary medicine in supportive cancer care models across four continents. Med. Oncol. 30, 511 (2013).
100. The Society for Integrative Oncology [online], (2013). http://www.integrativeonc.org/
101. Cassileth, B. R. et al. Complementary therapies and integrative oncology in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edn). Chest 132, 340S–354S (2007).
102. Deng, G. E. et al. Complementary therapies and integrative medicine in lung cancer: diagnosis and management of lung cancer, 3rd edn: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143 (Suppl. 5), e420S–e436S (2013).
103. Deng, G. E. et al. Evidence-based clinical practice guidelines for integrative oncology: complementary therapies and botanicals. J. Soc. Integr. Oncol. 7, 85–120 (2009).
104. Meyer, F. et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int. J. Cancer 122, 1679–1683 (2008).