Flexibility and goodwill have allowed patient involvement in the work of Europe’s drug regulators to develop at an impressive pace. But will they be enough to withstand the strains on the relationship exerted by financial pressures, together with demands that patient reps stop seeing industry reps?
Ten years ago, an opportunity arose for Hildrun Sundseth to help develop the patient voice in the European Medicines Agency (EMA), the EU agency responsible for the evaluation of medicinal products. She had long advocated for women’s health and saw this as a chance to change things gradually for her cause.
“Women are very much underrepresented in clinical trials,” says Sund-seth, who was the head of EU Policy at the European Cancer Patient Coalition at the time. This stems from the thalidomide tragedy when they “discovered when a woman is pregnant, the drug can be dangerous for the infant and cause birth defects”. Banning women from taking part in clinical trials for their own protection was a natural reaction, she explains – but in real life, women take medicines.
When the EMA set up the Patients’ and Consumers’ Working Party in 2006, Sundseth says that they “started with a clean sheet of paper”. Together with her fellow patient representatives, she contributes real life experience, which she says is very important. This includes “what it feels like [to live] with a disease as well [the impacts of] the medicines you are taking”.
The number of patients and consumers involved in EMA activities increased eight-fold between 2007 and 2014, from 76 to 633, according to the EMA’s latest report. Sundseth, who is now the President of the European Institute of Women’s Health, says this reflects the patient movement generally, but adds that “it’s amazing what progress has been made.”
This progress includes patient representatives being involved “across the whole medicine lifecycle”, according to Nathalie Bere, patient relations coordinator at the EMA. It’s been a journey, she says. What started as opening a dialogue has now transformed into involving patient representatives as an integrated part of their work.
“A lot of people didn’t really know at the beginning what to expect, having the patient around the table when it is predominantly a scientific discussion.” The approach was to start slowly and involve patients bit by bit into the different activities.
Now, patient representatives are voting members in most committees. So far, however, they have no seat at the table where the recommendations on marketing approval and other issues are decided – the Committee for Medicinal Products for Human Use (CHMP) – and that is where their sights are now set.
“We would very much like to be part of the CHMP,” says David Haerry, an HIV/AIDS patient advocate who co-chairs the Patients’ and Consumers’ Working Party, adding that, “In other committees the patients are very much part of the game.” Sundseth agrees. “We’ve pushed for a very long time to get involved in the CHMP,” she says.
As Bere points out, patient representatives already have some input into CHMP assessments via their involvement in scientific advisory group meetings, which are convened by the CHMP (see panel overleaf), but the EMA is open to discussion on how to improve the process.
A pilot is currently underway in which patients take part in the CHMP process as experts on benefit–risk assess-ments, and other ways of consulting patients through the CHMP are also being explored. Haerry expects this trial period to be over next year, after which the interaction will be analysed to see how to move forward.
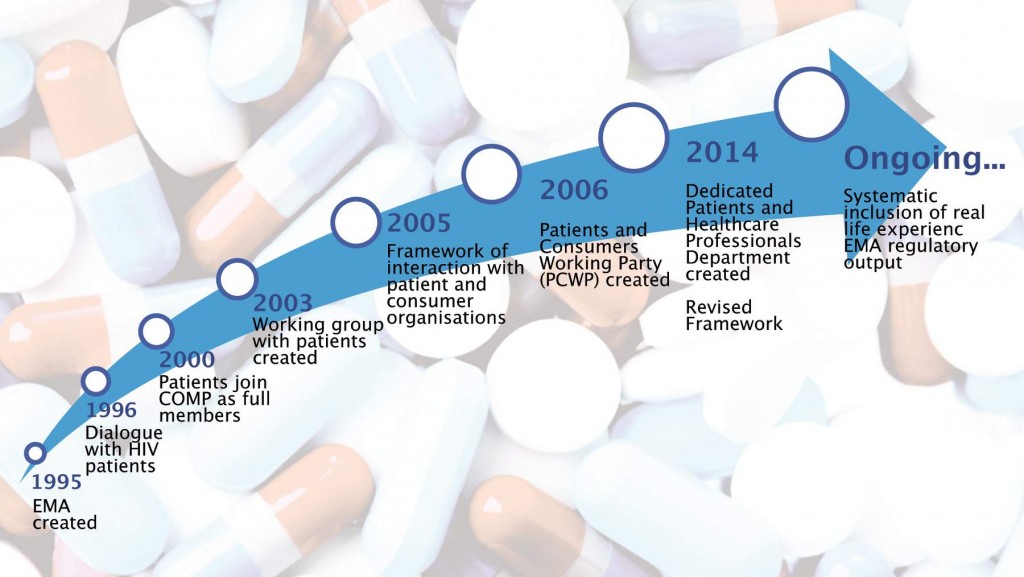
Source: European Medicines Agency
Penalties of participation
Achieving high-level input into EMA’s decision making processes is not just about getting formal access, however. There is also the problem of financial sustainability for the patient representatives who bring their knowledge and expertise to the table, and this problem is growing more acute as the extent and level of their involvement increases.
“Patients contribute mostly as volunteers, which means we are not paid,” explains Haerry. “It is an issue when you are part of a busy committee like the Pharmacovigilance Risk Assessment Committee.” He has calculated that patient representatives need to spend between six and eight days per month to keep up-to-date with the workings of such a committee.
Currently patient representatives are compensated for expenses such as travel and meals, but not for lost time. Haerry, who has been involved in the EMA since the inception of the Patients’ and Consumers’ Working Party, says “you cannot expect people to do this for free,” but adds that no solution has been found to date.
“This is something that we’re aware of,” says the EMA’s Bere. “We rely very much on voluntary work from a lot of patients, and it is obviously very much appreciated.” She adds that the EMA are constantly investigating to see if there are ways to compensate patient representatives further, but there’s nothing concrete as yet.
The European Institute of Women’s Health is campaigning for patient organisations to be funded for their work. Sundseth argues that they are providing a public service, and sees the lack of funding as a legacy issue. When the EMA and European Parliament proposed the amendment to consult patient organisations in the process, she says, they didn’t think about how to do it, and “there is no funding for these patient organisations to interact with the agency,” as a result.
This is not an issue in some other jurisdictions. The US regulators, the FDA, pay a salary to patient representatives for the time they spend in meet-ings, in addition to paying for expenses. Most members of FDA advisory committees are appointed as ‘special government employees’. The EMA can’t pay patients, according to Bere, because “we don’t currently have the legal basis for that.”

To resolve this issue, a change in EU legislation may be required. Sundseth says this will most likely be at least a five-year process, but she feels “that’s something we could do.”
Lack of funding not only affects individual patient representatives, who give up their time to attend meetings or committees, but also patient organisations who work or want to work with the EMA. “If you want to have total transparency, and patient organisations that are independent from industry, you have to support them with funding,” argues Sundseth.
Currently, there is a set of eligibility criteria against which patient and consumer organisations must be evaluated before they are eligible to work with the EMA. The criteria, as spelt out in a recent EMA report, include a “limitation of the amount of funding that organisations can receive from a single pharmaceutical company, the publication of their yearly financial accounts and adherence to a code of conduct/rules with regards to the relations of an organisation with industry.”
The lack of EMA funding can make it hard to meet the criteria, particularly for small, relatively new organisations, such as Melanoma Patient Network Europe. Bettina Ryll, who founded this network three years ago, says that ideally their funding would be split evenly between the regulator, pharmaceutical companies and health-technology assessment bodies, but this is not possible as the EMA doesn’t fund advocacy.
Since Ryll founded the new network, she is “personally no longer eligible” to take part in certain EMA activities, due to industry funding. “We all have the problem that we rely on pharma funding. It’s nearly impossible to get other funding, especially as a starting non-profit.”
Haerry, the Patients’ and Consumers’ Working Party co-chair, says that this is an ongoing discussion, and feels “it has taken some unfortunate turns.” Transparency is essential, he says, but he points out that EMA rules about interaction between patient representatives and pharmaceutical companies also mean that those who do work on EMA committees often have to restrict the advocacy work they do outside of the EMA.
He cites his own local work as an example. “In Switzerland, I work on a few things that nobody else in my country works on, so it is important that I can do my local work while being at the agency.”
Under the current criteria, however, he cannot attend local advisory meet-ings with industry, which means an opportunity for patients’ interests to be heard and taken on board by industry is being missed.
This may not be an issue at the European level, as other representatives can attend, but it is certainly a problem at country level. “Some of our patient [representatives] are quite rare birds,” Haerry points out.
He argues that the European Commission either needs to start funding patient organisations or the overall eligibility criteria need to be looked at again. “As long as the information is out and the organisations are transparent, I don’t really see this [as an] issue.”
Patient representatives and the CHMP
The Committee for Medicinal Products for Human Use (CHMP) sets up scientific advisory groups, as and when they may be required, to provide advice in connection with the evaluation of specific medicines or treatments.
These advisory groups are made up of European experts, including patient representatives. In recent years, almost all scientific advisory group meetings (82% in 2013) included at least one patient representative.
The eight scientific advisory groups currently running encompass areas such as oncology, vaccines, neurology and cardiovascular issues. They are convened for a variety of reasons including where issues may be controversial or involve complex technical assessments, or where major public health interests are
expected.
For oncology, typical questions that scientific advisory groups are asked to consider include:
- Whether benefit–risk is negative or marginally positive
- How clinical meaningful are the benefits
- The clinical impact of risks
- Need for further studies
- Guidelines
Patient interactions at these advisory group meetings have proved useful, according to the EMA. They provide a patient perspective to discussions about a medicinal product as well as providing insights into acceptable levels of associated risks. A 2011 survey of patient representatives showed that almost all felt their views were taken into account, and were able to follow the discussions at the meetings.
In September 2014, a pilot project was launched by the EMA to involve patients in the CHMP’s assessment of the benefits and risks of medicines. This pilot is intended to mark the next step in bringing patients’ views and values to the assessment of medicines throughout their lifecycle.
The first medicine to be included in this pilot was Scenesse, a treatment for erythropoietic protoporphyria, a rare genetic blood disorder that causes intolerance to light. During the approval process, two patients shared their experiences of living with the condition and answered questions from the CHMP. Their inputs were considered by the CHMP as part of its assessment of the treatment.
To date, there have been three cases of patient involvement as part of this pilot. A recent CHMP report said that “involvement of patients has been a learning curve and has improved with experience.” It added that, to complete the pilot, at least two to three more cases are needed before being able to deliver a sufficient analysis.
A flexible approach
EMA’s Bere says that patients are considered to be experts, and every expert coming to work at the EMA has to declare their interests and have these looked at prior to being involved. She stresses, however, that there are flexibilities. “It depends which activity you want to take part in, and what kind of interactions [with industry] there have been.”
For rare diseases, where patient advocates can be particularly thinly spread, there is also an option, which they call an ‘expert witness’. This allows patient advocates who have some interactions with industry to come and participate in a discussion and to share their views, with some restrictions.
Even when patients are ruled out due to conflict of interest, or their organisation is deemed ineligible, it is still possible to have some interaction with the EMA, and also take part in certain projects. One such example is a benefit–risk pilot, which is being undertaken by Melanoma Patient Network Europe.
“We have established a really constructive relationship [with the EMA] based on this pilot project,” explains Ryll. The network’s relationship with the EMA began when Ryll invited them to a conference on adaptive licensing, a new way to approach clinical trials for cancers such as stage IV melanoma.
Her interest in this step-by-step approach to licensing began when her hus-band took part in a number of randomised trials before he died of melanoma. Ryll says these trials were violating the Helsinki Declarations governing ethical research, as “one arm was better than the other” – the experimental treatment having already been shown to be highly efficacious at the phase 1 stage. She feels that, “from a patient perspective, that’s obviously not acceptable.”
Ryll organised an initial conference on clinical trial designs in 2014 (http://tinyurl.com/trials-we-want). Since then, she says, the Network and the EMA have “achieved a really good mutual understanding”.
It was this relationship that led to the benefit–risk pilot project, which involved a survey of patients with stage IV melanomas, carers and advocates. It also included a group of regulators.
“We got some quite interesting preliminary results, and based on that we decided to make it into a full project,” explains Ryll. The initial findings of the pilot showed that patients are much more willing to accept risk than either carers or advocates. “It actually turned out that advocates were more risk averse than regulators,” which Ryll feels has had a big impact on those who participate in the EMA.
Meaningful involvement
Francesco Pignatti, who is head of oncology, haematology and diagnostics in the EMA, has seen first-hand the extent to which patient involvement has had an impact on decisions. Though he can’t give specific examples, as discussions happen under confidentiality, he says that patient contributions have changed the outcome of benefit–risk assessment and risk minimisation measures in some instances.
A recent EMA report states that 40–50% of patient input is included in the final advice letter. As the report explains, while these figures are impressive, they “do not capture the benefit of each occasion where patient input has led to relevant interesting discussions or been in agreement with the advice provided by the working party.”
The creation of a network of young patient experts is the result of one of these interesting discussions at the Patients’ and Consumers’ Working Party. At an EMA meeting, Rafal Swierzewski, a fibrosarcoma survivor and board member of the European Cancer Patients Coalition, talked about the work ECPC was doing with young cancer patients, especially teenagers and young adults, to set up an advisory group within the Coalition.
The discussion led to Swierzewski being asked to help set up a similar advisory group within the EMA, and he is now involved in the creation of a new patient network. “The EMA like innovative projects,” says the cancer advocate. He welcomes the support he is getting from the regulatory body, with three EMA committees having now expressed their full backing for the youth programme. “It is important for me that the project has such a great support,” he says.
The EMA’s Bere feels that, “if the medicine [is] for young people, then we should be speaking to them as the end users.” She says that they are in early dis-cussions at the moment, and hope to set up a framework where teenagers can potentially be consulted.
There are some issues to work through, such as the legal aspects of involving minors, but Bere says they are looking into that. They are also trying to work out the best way to interact with young people by “trying to set up a network, so that we have contact with youth groups across different disease areas in Europe.” Social media, facetime, and video conferences are all communication methods currently under discussion.
Swierzewski says it is important that this new group of patients is involved, because their experience is often neg-lected. His past work in a children’s cancer organisation in Poland led him to witness many conversations between children as young as five.
“They were exchanging perfect professional information about their state of health in the corridors,” says Swierzewski, who argues that young people have an amazing knowledge about their disease and treatment. “But of course it’s never heard, as they only exchange the information between themselves.” This is what Swierzewski hopes to change, with the help of the EMA.
One change that is soon coming into force, involves the cause that Sund-seth, long-time patient advocate and policy expert, championed for a number of years. This May, a new EU clinical trial regulation (EU No 536/2014) will be implemented, and this will ensure that women will be included in statistically significant numbers in clinical trials.
It’s a big step forward, but Sundseth already has her eyes on the next improvement: the use of medication in preg-nant women. In her previous job at the ECPC, she often spoke to newly diag-nosed pregnant cancer patients who were worried and stressed because no information was available on the safety of their treatment options. “This is an area that we need to make headway in,” she insists. This means her involvement in the EMA is far from over.
So what else is in store for patient representatives at the EMA? Nathalie Bere says they are “talking about trying to consult patients even earlier in the assessment process.” Francesco Pignatti says that “more and more involvement is the natural evolution, which is still continuing.” David Haerry is determined to make sure that it does – as he says, “There’s more to be done.”




Leave a Reply