The chances of surviving a childhood cancer have changed very little over the past two decades. Sophie Fessl talked to parents, doctors, regulators and researchers about what has to be done to address this disastrous impasse.
“It feels odd to say this, but Elliot is one of the lucky ones.” The comment comes from Nicole Scobie, and refers to her son, who was only four years old when he was diagnosed with a stage IV Wilm’s tumour. His left kidney was engulfed by a huge cancerous mass and his lungs were full of metastases. The heart-stopping diagnosis was just the start of a rollercoaster of emotions, hospital stays and exhaustion. But this is a story with hope: Elliot responded well to chemotherapy. He went into remission after 10 months of treatment, and has remained so for the past four years.
“At least for his cancer, there is a treatment that works,” says Nicole. Not all the children she and her son befriended during their long stay at the Lausanne University Hospital’s children’s cancer ward can consider themselves as ‘lucky’. Elliot became close friends with Zoe, a little girl battling an aggressive neuroblastoma. But while Elliot’s prospects looked good, for Zoe, the odds were stacked against her. In the end, there was nothing her medical team could do: Zoe died in her mother’s arms aged four.
The difference between Elliot and Zoe? Elliot had a type of cancer that has been successfully treated for decades, being one of the first childhood cancers – alongside acute lymphoblastic leukaemia – to benefit from the chemotherapies pioneered by Sydney Farber back in the 1950s. His is part of the celebrated success story that saw cancer survival rates among children increase from 10% to 80% over 50 years.

Zoe, by contrast, had a type of childhood cancer that remains fatal in the majority of cases. Her story is shared by 6,000 children and young people under 24 who are still dying of cancer each year in Europe. For parents like Nicole Scobie, who’ve seen their child’s life in the balance, that is a heartbreaking statistic. But there’s a worse one – the mortality rate from childhood cancers has barely changed over the past 16 years.
Last year, Scobie was one of a large group of parents and advocacy organisations that got together to found Unite2Cure (unite2cure.org), an advocacy organisation that aims to kickstart progress again. It looks particularly to work with key players in the drug development ecosystem to improve the efficiency of developing new therapies for childhood cancers. A key focus will be the EU Paediatric Medicines Regulation, which came into force in January 2007 to try to address the obstacles to developing new drugs for children, and which is up for revision in 2017.
The Paediatric Medicines Regulation
The 2007 Paediatric Medicines Regulation sought to boost the development of drugs for use in children through a combination of obligations and rewards. Companies are required to discuss the potential for use in children of every drug they develop, and where appropriate to agree a Paediatric Investigation Plan (PIP) with the European regulators, the EMA. The results of studies carried out according to the PIP have to be included as part of any application for marketing authorisation for the new drug, unless the studies with children are not yet completed or were not required at all. In return for carrying out these studies, companies get an extension on their patent protection.
“The mortality rate from childhood cancers
has barely changed over the past 16 years”
Under the regulation, 48 new anti-cancer drugs for adults have come on the market – and six for children. Gilles Vassal, president of the European Society of Paediatric Oncology (SIOPE), welcomed this as important progress, but argues that much more needs to be done, and much faster.
“The paediatric regulation has definitely changed the landscape for drug development. The situation now differs positively from that before 2007. We have more access to new drugs and clinical trials. However, the regulation does not address the needs of children with cancer adequately. Major breakthroughs have been achieved in cancer care for adults, and these have not translated into breakthroughs for children.
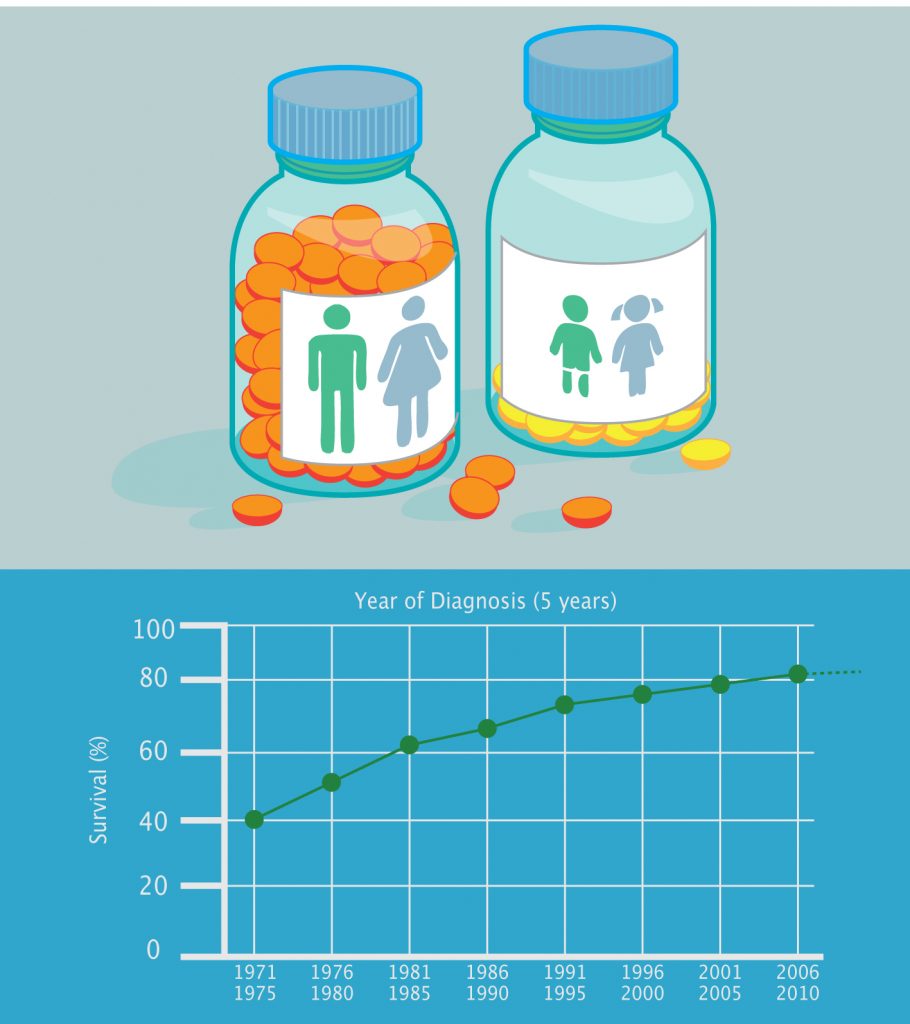
Source: Survival figures come from www.cancerresearchuk.org and are for the UK
“Cancer is still the number one cause of death by disease beyond one year of age. Less than 10% of children with life-threatening forms of cancer have access to new compounds. To increase survival, we need changes to accelerate the development of, and access to, new drugs,” he says.
Nicole Scobie shares his sense of urgency. “We parents just want our children to live. We are willing to do anything to get there. But the problem is that there is still not enough research, not enough drugs and not enough options. At Unite2Cure, we are calling for very specific changes to the paediatric regulation to harness the major advances made in adult cancer treatments for children.” As of August 2016, more than 2,700 supporters have signed Unite2Cure’s petition.
Academics are also uniting to call for improvements in treatment. Three years ago, the Cancer Drug Development Forum (CDDF), whose mission is “to facilitate interactions between all stakeholders to improve the efficiency of cancer drug development,” set up a CDDF-Paediatric Platform to promote discussion in preparation for the 2017 revision of the paediatric regulation. This was done in partnership with a variety of groups including the European Consortium for Innovative Therapies for Children with Cancer (ITCC), the European Network for Cancer Research in Children and Adolescents (ENCCA), the European Society of Paediatric Oncology (SIOPE), regulators and industry, as well as advocacy groups such as the Unite2Cure movement.
Ending the class waivers loophole
Unite2Cure and the CDDF are both calling for a much greater focus on biology in strategies for developing drugs for childhood cancer. This is partly to close a ‘loophole’ in the current regulations that allows companies to seek exemptions from testing and developing adult drugs in children on the grounds that the drug is intended for use in treating a disease that only occurs in adults – such as prostate cancer – even if there is a biological rationale to believe it could be of value to some childhood conditions. “Indication-based approval makes sense, for example, for drugs treating Alzheimer’s, as we don’t want to subject children to unnecessary trials,” Nicole Scobie points out, “but in cancer, the name of the cancer doesn’t matter. It is the biology that counts.”
The ALK gene is a case in point. This gene is implicated in a small minority of non-small-cell lung cancers, characterised by a MET-ALK dislocation. It is also implicated in several childhood cancers, such as some lymphomas (characterised by an NPM-ALK dislocation), some neuroblastomas (which have a mutation within the ALK gene itself) and a subtype of soft tissue sarcomas.
In 2012, an ALK-MET inhibitor, crizotinib, was approved for the treatment of ALK-positive lung cancer. However, as lung cancer does not occur in children, the developers had applied for, and received, a class waiver to exempt them from having to test the drug for use in children. This waiver was given by the EMA in 2010, a year after the company had been mandated to carry out paediatric development studies of crizotinib in the US, the results of which have since shown responses in children with lymphoma and sarcoma.
“This situation is paradoxical,” says Scobie, “considering that 90% of the drugs used for treating children with cancer in the past 40 years were originally developed to treat adults, often for a different cancer condition.” Unite2Cure is now demanding that the provision for class waivers be revoked as part of the revisions to the Paediatric Medicines Regulation.
They may be pushing at an open door, at least as far as the regulators are concerned. The EMA themselves do not appear to be happy with current progress in developing paediatric cancer drugs. “We share patients’ perspective that not enough has happened in terms of completed trials and approved drugs,” says Ralf Herold, Senior Scientific Officer at the EMA. “I fully understand that they are impatient. The clear progress for adult patients with cancer is not reaching children. A drug’s mechanism of action has been considered in all our discussions with companies since 2008. When we at the EMA see where and how a drug could be used in children, we flag it up to the companies developing the medicines. In fact, mechanism of action is also relevant for other areas of paediatric drug development. However, the EMA can only encourage, not force, companies to develop drugs for children based on their mechanism of action.”
Jeffrey Skolnik, Vice President Clinical Research at Tetralogic Pharmaceuticals and member of the CDDF, sees two reasons why pharmaceutical companies may feel reluctant to develop paediatric drug programmes. “Paediatric diseases are thankfully rather uncommon, and very few children develop cancer. It is therefore hard to invest in a paediatric drug programme: return on investment is low, but costs may not be lower. Pharma is a for-profit industry, and we need to provide financial return for our stakeholders. Secondly, children have historically been perceived as especially vulnerable. Companies are therefore very hesitant to dose children with experimental drugs.”
He recognises, however, that something has to change. “For cancer, this approach is not working.”
Prioritising the most promising
While closing the class waiver loophole may be seen as a priority by clinicians, researchers and advocates, ironically perhaps they also fear the reverse problem: too many companies chasing too few patients for their paediatric trials. Childhood cancers are rare, many of them very rare, which means that there aren’t a lot of patients to go round.
“With almost a thousand new drugs being developed in adults with cancer, we cannot study all of them in children,” says Vassal, who argues that prioritisation is key. “We need to find a way to choose the best drugs among the pipeline of all companies, taking into account their mechanism of action and the feasibility of trials,” he says.
“The clear progress for adult patients with cancer
is not reaching children”
This view is shared by at least some in the industry. Tetralogic’s Skolnik argues that “Drug prioritisation as a way to decide which company should move a programme forward – and which shouldn’t – could result in better use of resources, especially if we take a mechanism of action approach for cases where a minimum of preclinical data or adult data are available. If we optimise, prioritise and divvy out responsibilities, we can focus on the most likely successes rather than testing every single option.”
The EMA’s Ralf Herold sees the issue in a rather different light. “Actually, prioritisation is not yet needed. So far, we don’t have enough drugs developed for children or studied as it is. We would rather like to see more relevant drugs studied, and work on an approach to get more medicines into trials for children. But we haven’t lost hope yet – maybe prioritisation will become an issue [with the revision of the regulation] after 2016.”
Uncoupling development for children and adults
As much as the Paediatric Medicines Regulation may have changed the landscape for drug development for children, the elephant in the room remains: what can be done to promote development of drugs exclusively to treat children with cancer?

The PIP process treats drug development for children as an extension of adult drug development. It does not encourage testing of specific drugs for childhood-only indications. Indeed pharmaceutical companies may choose to abandon PIPs even if positive responses are seen in children, in cases where the adult trial is unsuccessful, as happened with IGFR-1 inhibitors.
Rather than relying entirely on ‘big pharma’, there could be a case for looking to small biotech companies to play a key role in developing new paediatric oncology drugs – companies such as the Vienna-based start-up Apeiron Biologics. Their lead project is an antibody, dinutuximab beta (APN311), which has already been submitted for marketing authorisation in the EU for the treatment of neuroblastoma – the cancer that killed four-year-old Zoe.
Dinutuximab beta offers an interesting model for how cooperation between academia and companies might bring new drugs to children with cancer. Originally, APN311 was studied exclusively by academic researchers, for the European market, with funding by European charities. Apeiron Biologics then picked up the development and took it further to submission for marketing authorisation.
CEO Hans Loibner, believes this project sets a precedent: “Initially, we were interested in APN311 because it was a cancer immunotherapy already in clinical trials rather than because it was a medicine for paediatric cancer. But our work has shown us that it makes sense to develop medicines for children with cancer. This project is worthwhile ethically – we help seriously ill children – and the project for us is commercially reasonable.” The company, which has several drugs already in their pipeline, is committed to developing more drugs for treating childhood cancers, he says.
Loibner believes small biotechs may be particularly well suited to developing drugs for small patient populations. “We develop drugs smarter, more streamlined than big companies,” he says. “Usually, this line of development is not interesting for pharma because investment is high and the market small. A reasonable sales prediction for our neuroblastoma treatment is in the range of €100 million turnover worldwide. This may not be enough for a big pharma company, in which drug development is much less flexible. But the support received for developing orphan medicines, together with the prices that can be achieved, make it an attractive model for small companies. I believe that in the future, orphan drug development will be a domain for small and mid-size companies.”
New incentives
As Loibner points out, the attraction and viability of developing drugs for childhood cancers depends in large part on the incentives offered to compensate for the small market size. Tetralogic’s Skolnik is developing ideas on how this should be done, as part of a CDDF working group.
The discussion, he says, centres around risk-sharing models, which provide earlier, up-front awards for developing paediatric programmes. “These could be based on stratified PIPs that segment the drug development process. If, for example, a phase I study with efficacy test is performed to satisfy the first part of a PIP, the company could receive some investment as reward, such as a few months of patent extension.”
Creating new incentives to encourage the development of specific paediatric oncology drugs is one of the aims of Unite2Cure and the CDDF Paediatric Oncology Platform. One idea comes from the US, where the Creating Hope Act of 2011 encouraged the development of three new paediatric oncology drugs through new market incentives.
“A multi-stakeholder approach
is starting to happen,
but it is not crystalising into results”
The Creating Hope Act provides a ‘priority review voucher’ to companies that develop drugs specifically for serious and rare diseases, including paediatric cancers. This voucher can be used to secure expedited approval of any drug, not just for rare indications. As the priority review voucher can be sold to another company, using a system somewhat analogous to the carbon emissions trading scheme, market value is created even for smaller companies with very limited drug pipelines.
The voucher given for Unituxin under the US Creating Hope Act shows how much value they carry: originally received by United Therapeutics for its neuroblastoma treatment, in 2015 the company sold the voucher to AbbVie for $350 million.
Can paediatric review vouchers work in the European market? All stakeholders agree that new incentives need to be suited to the European reality. The EMA’s Herold argues that additional tools may be needed for stimulating the necessary research: “Paediatric review vouchers can be applied if the paediatric development of a drug is successful. However, studies showing that promising drugs eventually turn out not to be efficacious, or are not safe enough, are also important research.” The ideal incentives, he says, would be related to the quality of research carried out.
Skolnik sees new models of cooperation and a multi-stakeholder approach as key to incentivising paediatric drug development: “Currently, the burden in drug development falls on the pharma industry in terms of time, resources and money. New incentives could share this burden with different stakeholders, including academics and people passionate about raising money, to de-risk paediatric development.
For example, companies could put in the research and provide the compound, while foundations may invest money, so that the for-profit organisation performs a study it otherwise would not do.”
One thing everything seems agreed on is that urgent changes are needed to the way the EU regulates the development of paediatric medicines. Jordi Llinares Garcia, who heads up the EMA’s Product Development Scientific Support department, puts it this way. “Much has been achieved since the regulation came into force. There has been a significant change in the focus of companies on paediatric development as an additional line of research. But we are not there yet – much remains to be done for children with cancer. A multi-stakeholder approach to drug development for childhood cancer is starting to happen, but it is not crystalising into results. We need to take another step: to deliver and actually improve outcomes.”
Nicole Scobie, and the advocacy movement she works with, are determined to see that step taken, and soon. “I don’t want to watch any more mums lose their child. I don’t want to hear any more dads talk about their daughter in the past tense. I can’t. I won’t.”
Cancer World journalists’ grant scheme
This article originated in a proposal submitted by the author for consideration for a Cancer World journalists’ grant, a scheme set up to encourage journalists working in print, broadcast or online mass media to tackle more complex, multi-source, analytical articles that explore systemic issues that have an impact on patients.
Further information about the grant can be found at https://archive.cancerworld.net/media/cancerworld-journalist-grant/





