An exercise programme can improve patients’ physical ability to function, lift their mood and may even lower their chance of recurrence.
 Four million people will be living with a diagnosis of cancer in the UK alone by 2030 (www.macmillan.org.uk). This is equivalent to more than 7 out of every 100 members of the adult population – a proportion that will be reflected across much of Europe, where incidence and survival rates are broadly similar. The challenge in ensuring that these survivors enjoy a good quality of life is to address the chronic or late-appearing side-effects of cancer and cancer treatments. These include fatigue, which is one of the most debilitating long-term effects of cancer; weight changes (either weight gain or loss); loss of bone density, including osteoporosis; cardiotoxicity; lymphoedema; psychological problems including anxiety, depression, fear of recurrence and cognitive dysfunction; and limited range of movement.
Four million people will be living with a diagnosis of cancer in the UK alone by 2030 (www.macmillan.org.uk). This is equivalent to more than 7 out of every 100 members of the adult population – a proportion that will be reflected across much of Europe, where incidence and survival rates are broadly similar. The challenge in ensuring that these survivors enjoy a good quality of life is to address the chronic or late-appearing side-effects of cancer and cancer treatments. These include fatigue, which is one of the most debilitating long-term effects of cancer; weight changes (either weight gain or loss); loss of bone density, including osteoporosis; cardiotoxicity; lymphoedema; psychological problems including anxiety, depression, fear of recurrence and cognitive dysfunction; and limited range of movement.
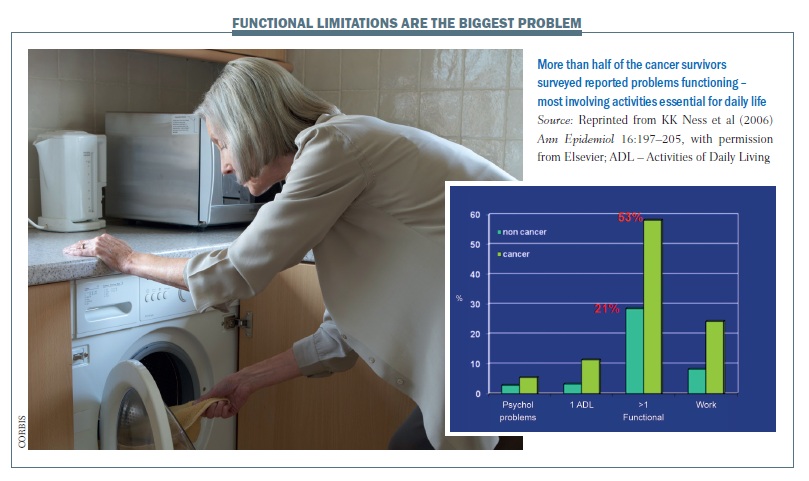
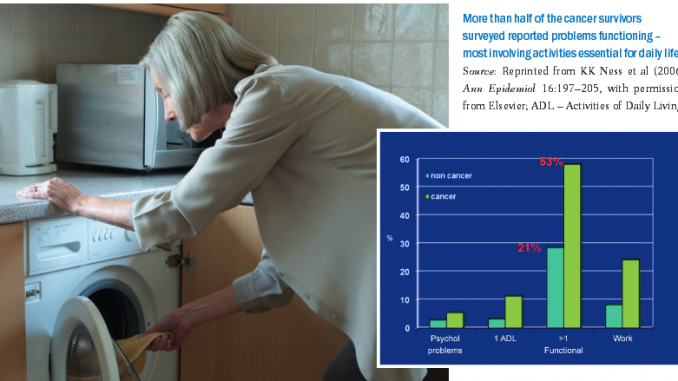
A US study showed that more than half of cancer survivors (53%) had problems functioning, with at least one functional limitation, compared to only 21% of people of the same age who had not had cancer (Ann Epidemiol 2006, 16:197–205). The study was undertaken with an elderly population, and the common functional problems were: crouching/kneeling; standing for two hours; walking quarter of a mile (0.4 km); lifting or carrying a load of 10 lb (4.5 kg); and standing up out of a chair. These movements are all the basis of daily activities needed for housework, shopping and similar tasks, suggesting that functional aspects of quality of life tend to be quite dramatically reduced after a cancer diagnosis.
Exercise-based cancer rehabilitation: the evidence
The number of high-quality studies in this field is increasing exponenti-ally each year. They show that physical activity can reduce the functional loss (cardiovascular and muscular) that occurs with cancer and its treatment, and can reduce some of the chronic or late-appearing side-effects of treatment, including fatigue, depression and weight gain. Exercise-based rehabilitation can also reduce some of the long-term reliance on health services. Emerging epidemiological evidence suggests that being physically active reduces the risk of the cancer recurring, as well as all-cause mortality, for breast, prostate and colorectal cancers. As rehabilitation programmes are already available for coronary heart disease, diabetes, preventing falls and the lung disease COPD, programmes for cancer survivors can tag into or copy programmes that have been tried and tested for other chronic conditions.
During treatment
A systematic review of high-quality, randomised controlled studies of physical activity as an intervention during adjuvant treatment for cancer – during chemotherapy, radiotherapy or after surgery – shows that physical function, in terms of cardiovascular function and muscular strength, improves significantly (grade A evidence; J Cancer Surviv 2010, 4:87–100).
The 17 randomised controlled trials all showed a small to moderate effect in improving cardiovascular fitness or muscular strength in patients taking part in a physical activity programme during adjuvant treatment.
Some studies have shown physical activity can reduce fatigue during treatment, but the overall effect is not very large. However, the good news is that being physically active during treatment does not increase tiredness. Anxiety, self-esteem, quality of life and depression show small improvements with physical activity during treatment. Also exercise interventions aid in significantly reducing body fat and increasing lean muscle mass. The overall take home message is that it is safe and effective to give an appropriate programme of physical activity while on adjuvant treatment. This can help prevent the functional decline that can occur during and after cancer treatment.
Question. There is a modest increase in physical function with physical activity. Do you have any information on age subgroups? Will a patient who is 80 years old benefit as much as a younger person with non-Hodgkin lymphoma at the age of 20?
Answer. The majority of studies mentioned in this report are with women with breast cancer with an average age of 50–60. I believe that a similar effect will be found irrespective of age, but perhaps a younger person with non-Hodgkin lymphoma would gain more fitness from a physical activity intervention, while a frail elderly person might gain more active daily living and confidence. The results will vary with the cancer type and the patient’s age and condition.
After treatment
What about giving an exercise intervention in a patient who has finished adjuvant treatment? Results here are more significant. Exercise interventions achieve large increases in muscle strength (effect size [ES] 0.90 in seven randomised controlled trials) as well as significant improvement in cardiovascular fitness (ES 0.32 in 14 RCTs). For many but not all patients, this timepoint, just after treatment, is when they are ready to start an exercise programme. Fatigue levels also show a significant reduction with exercise interventions. Wellbeing improves more significantly with activity after treatment, and there are small reductions in body fat and increases in muscle mass. It is important to note that these trials involved only an exercise intervention; studies incorporating both exercise and diet may give even better results for positive changes in body composition.
Finally, some new studies have looked at the effect of exercise on bone density. Reduced bone turnover can occur with hormonal therapies, potentially reducing bone density. Resistance exercise programmes can help reduce the risk of bone thinning, but the results of different trials to date are inconsistent.
Other benefits of exercise are being explored in relation to some of the late effects of cancer treatment. As well as improving bone health, studies have shown exercise programmes can improve range of movement. Activity programmes designed for individual patients may prevent lymphoedema, and studies have shown that exercise does not exacerbate lymphoedema in patients who already have the condition. Further studies have shown improved mood, better regulation of insulin, reduced cardiotoxicity and improved immune system function with exercise.
A smaller number of studies have looked at the effects of exercise in people with advanced or terminal cancer. A systematic review of six small studies investigating physical activity during palliative care showed some benefit. One study showed that home-based, seated exercises prevented decline in quality of life (Oncol Nurs Forum 2004, 31:977–983). Supervised group exercise for six weeks improved fitness, functional ability, emotional wellbeing, fatigue, dyspnoea and anorexia (J Pain Symptom Manage 2006, 31:421–430). The key message in a palliative setting is that the patient’s preference is important. The aim is not necessarily to improve fitness, but to maintain independence and wellbeing towards the end of life.
Impact of physical activity on cancer recurrence: the evidence
Kenfield and colleagues followed 2705 men diagnosed with prostate cancer over 10 years, monitoring activity levels. Results showed that the number of deaths in total was 36% lower in men who walked an average of one hour each day (7+ hours per week). It was 49% lower among men taking three or more hours of vigorous activity each week, with 61% fewer cancer deaths (JCO 2011; 29:727–732).
The strongest beneficial effects of physical activity on disease recurrence and risk of death has been observed in longitudinal studies of breast cancer survivors. A systematic review of nine high-quality, prospective cohort studies following breast cancer survivors over time showed that undertaking leisure-time physical activity involving moderate-intensity exercise for 30 minutes most days each week was associated with a 30% reduction in breast cancer mortality risk when compared to a sedentary lifestyle with virtually no physical activity (Maturitas 2010, 66:5–15; Med Oncol 2011, 28:753–765). Two longitudinal studies have shown that colorectal cancer recurrence risk and mortality is reduced by about 50% with six hours of moderate-intensity physical activity each week (JCO 2006, 24:3535−41).
Why might exercise be protective?
Research is starting to explore why people who are physically active may be protected from cancer recurrence. Exercise may have a beneficial impact on energy balance and fat distribution, and this is important as obesity is strongly associated with higher risk of cancer recurrence. Secondly, physical activity can influence sex steroid hormones, such as oestrogen and testosterone, which affect some hormone receptive cancers. Insulin, insulin-like growth factor and its binding protein IGF-BP3 seem to be involved in cancer cell growth, and their levels can be influenced by physical activity. Inflammatory markers (such as CRP and interleukins) and immune system components (such as natural killer cells) may also play a role in cancer and can be regulated by exercise. Finally, physical activity may impact on the antioxidant defence system, DNA damage and apoptosis.
What do guidelines recommend?
During cancer treatment (surgery, chemotherapy and radiotherapy), guidelines recommend that patients should exercise to tolerance (Med Sci Sports Exerc 2010; 42:1409–26). This means that interventions should be individualised for each patient according to how fit they were before developing cancer, their current treatment, and the side-effects they encounter. After treatment, and when patients are feeling stronger, they should try to accumulate about 20–30 minutes of moderate-intensity cardiovascular fitness training three to five days per week, and also incorporate muscular strength and endurance training twice a week. Activity to maintain balance and flexibility is also beneficial. Physical activity programmes should be individualised, based on a patient’s needs, goals and exercise preferences, and taking account of any barriers to exercise, including long-term side-effects related to their treatment and disease. Brisk walking is a great simple and easy cardiovascular activity that patients can do to get health benefits – even five to ten minutes’ walk round the block or to the shops can be enough to make a difference.
Question. Research shows a risk reduction of 30% or 40% with exercise for the three most common cancers, and this exercise does not need to be intensive but can just be walking for 20–30 minutes a day. To me that just looks amazing. It’s extremely cost- effective compared to the costs of cancer treatments. Why isn’t there a major programme in the UK or elsewhere to help cancer patients take exercise?
Answer. There are now a number of programmes that are beginning to emerge throughout Europe. Here at Dundee we have a programme called MoveMore, available for any person living with cancer – it offers home-based programmes with a DVD and booklet, one-to-one consultations, gym instruction and group- and water-based exercise programmes. A sample of approximately 12 different types of programme (e.g. hospital-based, home-based, individual, group, etc) is being evaluated by Macmillan, a UK cancer charity, for improvements in quality of life, active daily living and cost–benefit.
 Question to the live webcast participants. Patients at my centre have no formal access to exercise rehabilitation. Do yours?
Question to the live webcast participants. Patients at my centre have no formal access to exercise rehabilitation. Do yours?
Responses. Yes: 44%. No: 56%.
Question to participants. In your opinions, how many patients could benefit from such an intervention?
Responses. Most participants considered 30–50% of cancer patients would benefit.
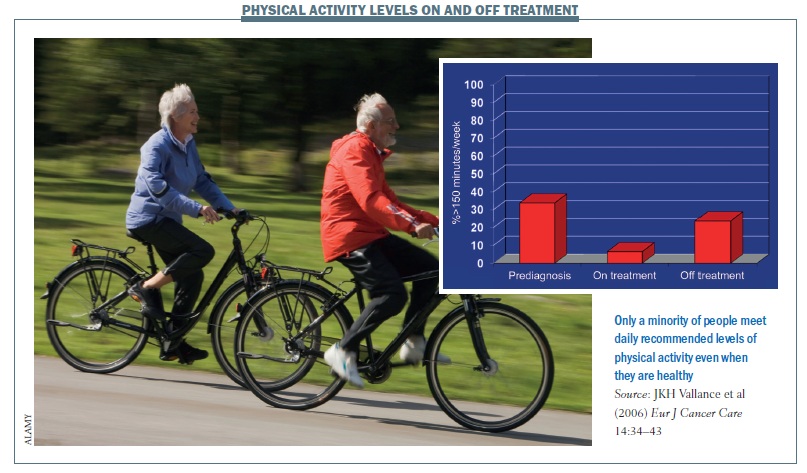
For a lot of people who have not participated in any structured physical activity for a long time, a cancer diagnosis can be ‘a teachable moment’ (JCO 2005, 23:5814–30). Being diagnosed with cancer can increase a person’s motivation to make lifestyle changes, including incorporating exercise into their lives. However, figures for the US – which are very likely to be true also for other countries – show the number of people doing the recommended level of 30 minutes moderate-intensity activity on most days of the week is very low (Eur J Cancer Care 2006, 14:34–43).
Only 30–40% of the population take the recommended amount of exercise before being diagnosed with cancer, and this falls to only about 5% of patients on treatment and 20% off treatment.
Putting the evidence into practice
In 2000, I carried out a small pilot study which showed that ‘rest is not best’ during breast cancer treatment, but that physical activity could improve quality of life. In 2003, my colleagues and I began a larger study funded by Cancer Research UK – the Glasgow Study – that randomised 203 women on chemotherapy or radiotherapy for breast cancer to attend group exercise classes (a combination of cardiovascular and strength training) twice a week for 12 weeks, or to the usual care group of no structured exercise. After 12 weeks, women taking part in the exercise programme (held in community-based settings) had improved significantly in walking pace, weekly activities, shoulder mobility, breast cancer specific quality of life, and positive mood. Six months later, women who had been randomised to the exercise intervention during treatment still had better overall quality of life in terms of physical functioning, positive mood, and less fatigue and depression than the usual care group (BMJ 2007; 334:517)
Analysing the cost-effectiveness, the NHS (National Health Service) cost of the intervention was £400 (€470) per woman for a safe and effective intervention that provided short- and long-term physical, functional and psychological gains. Participants spent fewer nights in hospital and had fewer GP visits than the usual care group, giving a saving of £1507 (€1750) per patient. Overall, the intervention achieved conventional standards of cost-effectiveness (unpublished data).
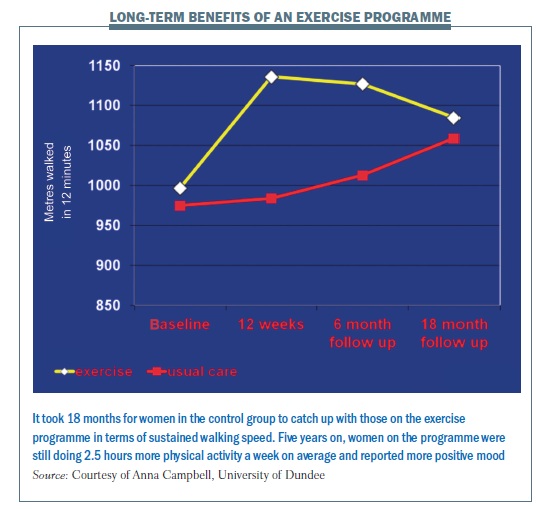
Following the women for five years showed those in the exercise group could walk much further in 12 minutes after the intervention, and this was sustained until the 18-months’ follow-up timepoint. For the 87 women (out of the original 203) who attended the five-year follow-up, those originally randomised to the exercise group still reported significantly more leisure-time physical activity (2.5 hours more per week) and a more positive mood than the control group. Those taking part in recommended amounts of physical activity, irrespective of original group, recorded a greater decrease in depression levels at all follow-up points. This showed that the 12-week exercise programme gives lasting effects, and staying active can reduce depression after cancer (J Cancer Surviv 2012, 6:420–430).
In light of the results of that study, the city of Glasgow has now introduced the Active ABC – Active After Breast Cancer – programme for any woman diagnosed with breast cancer in the past five years. It provides 24 free sessions led by trained fitness professionals followed by sessions on health behaviour change. The programme works by self-referral, but patients can be signposted by health professionals. A national vocational qualification has been developed to train people to become cancer rehabilitation specialists (www.canrehab.co.uk) and there are now more than 200 qualified cancer exercise specialists in the UK. Dundee University is setting up a cancer rehabilitation centre for training, research and practice. The aim, as mentioned earlier, is to make exercise-based rehabilitation a sustainable part of every cancer survivors’ care pathway.
 Summing up
Summing up
Keeping physically active after a cancer diagnosis appears to be a safe and effective way of improving physical, functional, social and emotional aspects of quality of life. Programmes should be individualised. The key message for patients is that a little physical activity is better than nothing, and they should avoid being sedentary. Right from their diagnosis, patients should have information that being active can be helpful and health professionals need to be aware of the strong evidence for the benefits of exercise. For the future, more research is needed on cancer-specific guidelines for physical activity, into mechanisms and into ways to improve behaviour change.