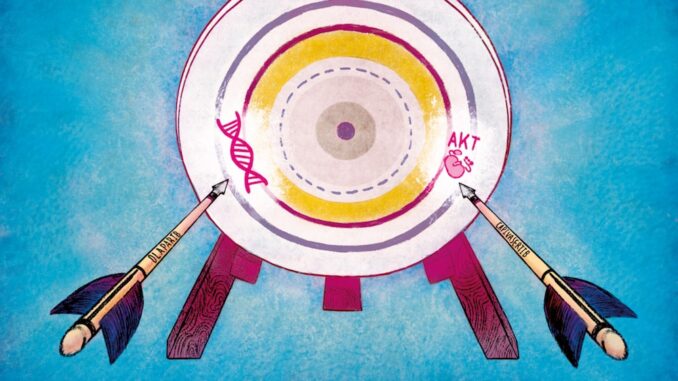
For the first time, an investigator initiated phase 1b study tested the combination of the PARP inhibitor olaparib, and the AKT inhibitor capivasertib, and showed this combination was well tolerated and achieved durable responses in patients with treatment-refractory breast, ovarian and prostate cancers. The results were published in the journal Cancer Discovery (Yap TA et al. Cancer Discov 2020).
“Our study establishes the potential of a combination of two precision medicines for patients whose tumours have stopped responding to existing drugs. The combination of olaparib and capivasertib works by attacking two fundamental weaknesses in cancer, and could potentially be used to treat patients with several common tumours,” explains Johann de Bono, senior author of the study from the Institute of Cancer Research and Royal Marsden NHS Foundation Trust, London.
The utility of PARP inhibitor monotherapy has been limited by the emergence of drug resistance and generally short-lived anti-tumour responses. The BRCA family of proteins are involved in DNA repair, but many cancer cells have mutations in BRCA1 or BRCA2. PARP (poly-ADP ribose polymerase) is important in DNA repair, and can be targeted by PARP inhibitors. When a BRCA deficient cell is exposed to a PARP inhibitor, this disrupts two of the most important mechanisms responsible for repairing DNA, leading to cell death in a process known as ‘synthetic lethality’. Cancer cells can escape the control of PARP inhibitors leading to the development of resistance. The AKT inhibitor capivasertib, which targets the PI3K-AKT pathway, can increase disruption of BRCA and thereby further destabilise DNA repair mechanisms and increase sensitivity of cells to PARP inhibition.
In this study from four major UK cancer centres, 64 patients with a range of treatment-refractory advanced solid tumours including breast, ovarian and prostate cancers, were recruited into the dose-escalation arm (n=20) or the dose-expansion arm (n=44). For the dose-escalation arm, patients received increase doses of capivasertib after each cycle in combination with a fixed olaparib dose provided no grade 2 or worse toxicities were observed.
Results showed that the combination was well tolerated, and that 45% of the patients whose response could be assessed (25 out of 56 patients) achieved clinical benefit (RECIST complete or partial response criteria or stable disease greater than four months). Treatment-related toxicities were reversible and mainly GI-related, including diarrhoea, mucositis, nausea, and anorexia.
The team are now planning further later-stage clinical trials to assess this drug combination in patients who do not have AKT aberration or DNA repair defects. The recommended phase II dose for the two schedules were olaparib 300mg twice daily with either capivasertib 400 mg twice daily four days on, 3 days off, or capivasertib 650 mg twice daily, two days on, five days off.
“The trial design used in the combined drug study is very innovative”, comments Aoife Regan, Head of the ECMC Programme Office at Cancer Research UK. In a traditional 3+3 dose-escalation study, starting doses are usually low and given to the first three patients to see how they react. If there is no toxicity, the dose is increased and given to the next three patients, and so on until at least two patients among a cohort of three experience dose-limiting toxicities. “This means that in the dose-escalation part of a conventional study the majority of patients don’t receive a potentially therapeutic dose level. However, with this methodology, a patient is given the first low dose, then in the same patient the dose is increased the next time a dose is due. The result is that more patients receive potentially therapeutic doses of the drug,” says Regan. She continues, “Patients have a variety of responses to these drugs, and we don’t know why. The level of molecular data for every patient in this study will help understand how these drugs are working in different patients, and so, how to improve combination strategies in the future.”
The Institute of Cancer Research says that the study represents ‘an exemplar’ of the type of research they will be undertaking in their new Centre for Cancer Drug Discovery, which aims to use combination treatments to overcome drug resistance.
Illustration by Sara Corsi





Leave a Reply