Information on how the performance of individual clinicians, teams and hospitals compares with the best is key to driving up standards in cancer care. ECCO is designing and implementing an audit framework named EURECCA to do so.
Improving the quality of cancer care through performance feedback to hospitals and healthcare professionals is a topic of growing interest for policy makers and cancer professionals alike. Well-performing health systems make the best possible use of finite health budgets. Monitoring performance across hospitals, health regions and countries also helps identify and spread best practice and can potentially save patients from being treated in places and by professionals who are not up to scratch.
But how can such monitoring be organised in something as complex as cancer care, and who should be responsible? These were two of the questions that were addressed at the ‘oncopolicy’ session on Inequalities in Cancer Care, organised at the ECCO conference in Stockholm last September.
Chairing the session, ESMO president David Kerr emphasised the need for effective audit systems. “In the bid to improve clinical standards it’s really important to develop systems which capture clinical outcomes and make them readily available to citizens,” he said.
The urgent need to harmonise cancer care throughout Europe, he added, is underlined by the results of successive EUROCARE epidemiological studies, which reveal persistent differences in cancer mortality between countries. Despite improvements in survival in eastern European countries due to better cancer care and screening, the east–west gap in Europe still continues. The EUROCARE-4 study, which was published in the European Journal of Cancer in 2009, covering 42 types of cancer in 23 European countries, showed that five-year relative survival for all cancers ranged from 58% in Sweden to 39% in Poland. Marked survival differences also exist among western countries, with survival in the UK and Denmark for several cancers being relatively low. Other publications on social inequalities and cancer have revealed large differences within western countries in both cancer incidence and mortality. In a study on educational inequalities in cancer incidence done in Turin, Italy, and published in the European Journal of Cancer Prevention in 2009, a low level of education was associated with higher risks of upper aero-digestive tract (UADT), stomach, lung, liver, rectal, bladder, central nervous system and ill-defined cancers in men, and with stomach, liver and cervical cancers in women.
The roots of inequity
In the key note presentation, Kathy Redmond, editor of Cancer World, took a closer look at some of the factors that contribute to such variations in incidence and outcomes. Differences in the level of public investment in health care (total health expenditure as a share of the country’s GDP), the technology available (such as number of MRI machines), access to specialist doctors and nurses, the number of hospital beds available and reimbursement systems all play a role. “The way cancer services are organised has a huge influence on patient outcomes and has the power to determine whether people live or die,” she said.
“The way cancer services are organised has the power to determine whether people live or die”
Redmond referred to the OECD’s draft report ‘What Explains Differences in Cancer Survival Rates?’ which was published in 2011. Based on extensive data gathering and analysis, together with questionnaires and interviews covering many cancers and countries, the OECD report concluded that around 50% of differences in survival rates can be explained by financial resources (such as access to drugs, number of oncologists, number of comprehensive cancer centres), 33% by process quality (i.e. whether populations have access to services such as cancer prevention, early detection and evidence-based cancer care) and 17% by governance – ensuring the whole system functions as well as possible. Drawing up national cancer control plans, introducing cancer-specific targets with timeframes, developing networks for service delivery, setting in place quality assurance mechanisms and monitoring progress, said Redmond, are all essential aspects of good governance.
The Ontario example
The Cancer System Quality Index (CSQI) developed and used in Ontario, Canada, offers a prime example of how good governance initiatives can improve service delivery by setting standards for care and measuring their implementation, said Redmond. Launched in 2005 by the Cancer Quality Council of Ontario (CQCO) – a quasi-independent body with a mandate to monitor and report publicly on cancer system performance – in partnership with Cancer Care Ontario (CCO) – a public (provincial) agency responsible for continually improving cancer services – CSQI now undertakes an annual audit reviewing the province’s progress against cancer.
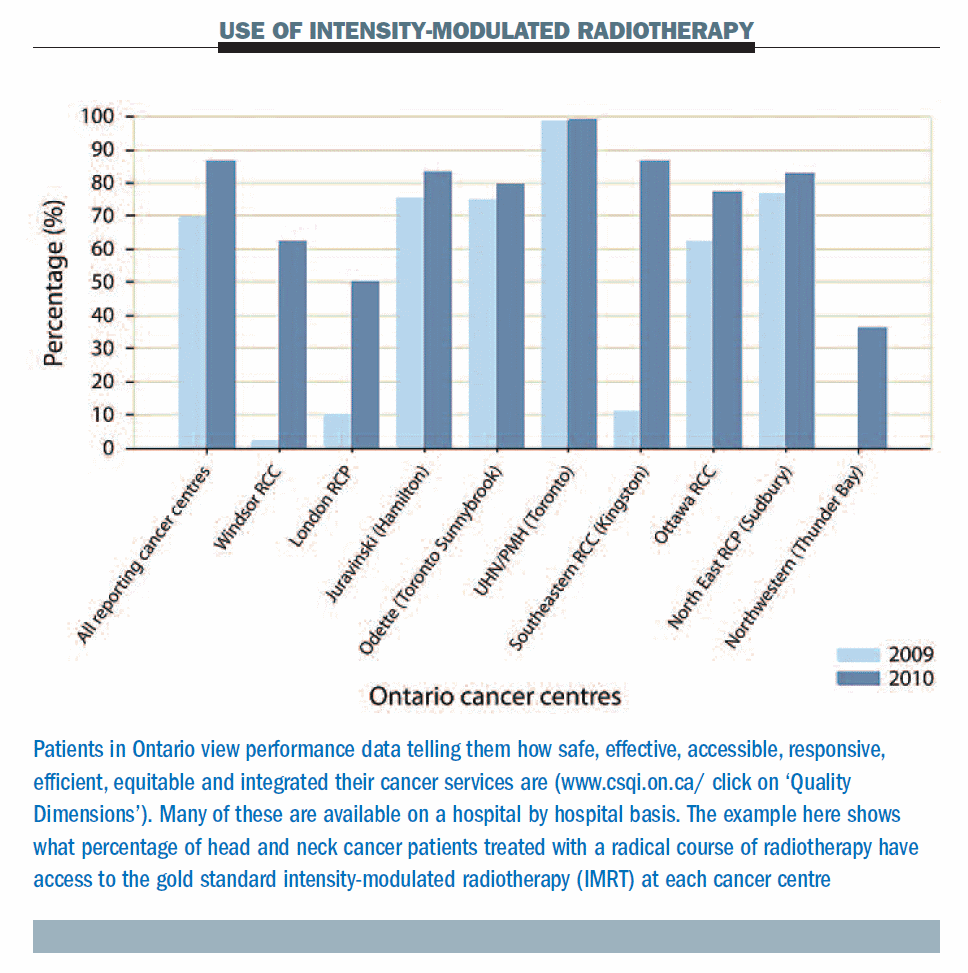
The audit reports on 36 evidence-based quality measures covering every aspect of cancer care from prevention to end-of-life issues. Measurements include the percentage of liver and pancreatic cancer patients whose surgery is done at designated referral centres, the percentage of patients who have their care discussed at multidisciplinary case conferences, and the percentage of patients undergoing a radical course of radiotherapy who receive the gold standard intensity modulated radiation therapy. Other aspects covered by the audit include waiting times between cancer surgery and adjuvant chemotherapy, the percentage of patients treated according to guidelines, and patient satisfaction with care, including their satisfaction with the emotional support offered by healthcare professionals, continuity of care and respect for their preferences.
Health professionals, cancer organisations, planners and policy makers can use the web-based CSQI audit results to identify cancer trends and plan improvements to the service. A significant rise in the proportion of lung cancer patients being screened for symptoms using the Edmonton Symptom Assessment System (a screening tool rating the severity of nine symptoms commonly experienced by cancer patients) is one example of an improvement that has been directly attributed to the CSQI. In 2008, when CSQI first began reporting on the use of ESAS, 43% of lung cancer patients were screened using the tool, but two years later this had climbed to almost 60%, with some cancer centres reporting that over 85% of patients had been screened. “The CSQI process helped highlight the value that patients place on the ESAS screening tool as a crucial component of their care,” explained a representative of CSQI later to Cancer World.
“Health professionals and policy makers can use the audit results to identify trends and plan improvements”
The variability of cancer incidence and mortality within and across European countries shows there is an urgent need for governments on this side of the Atlantic to follow the Ontario example, and establish clearly defined standards of care for cancer patients and then monitor and improve their delivery, Redmond argued.
EURECCA – a new quality framework
In the absence of the sort of public initiatives represented by the CSQI audit in Ontario, issues of audit and quality improvement in Europe have so far been largely left up to professional organisations. Bodies like EUSOMA and EMBT have introduced quality improvement initiatives in their respective fields of breast cancer care and blood and marrow transplantation, while the Organisation of European Cancer Institutes has implemented an accreditation scheme for cancer centres, with a commitment to continuous improvement among the assessment criteria. Now ECCO itself is introducing a new quality framework, EURECCA (European Registration of Cancer Care), which is kicking off with some core countries, focusing on care of colorectal cancer patients.
Speaking in the panel discussion, Cornelius van de Velde, president of ECCO, and a cancer surgeon at Leiden University Medical Centre, in the Netherlands, described how EURECCA will work, and outlined ECCO’s plans to develop it into an infrastructure where every cancer treatment centre across Europe can upload information about tumour characteristics, treatments, and patient outcomes onto a single site.
“The aim is to first identify best practice, and then use this information to shape guidelines that can spread the word throughout Europe. The approach can also provide performance feedback to individual centres,” explained van de Velde, speaking later to Cancer World.
By inputting data on the treatment and outcomes of multiple patients from centres across Europe, EURECCA should make it possible to tease out the approaches associated with the best overall survival statistics and least side-effects. Data from large numbers of patients would allow comparisons of ‘like with like’. “It’s only then that you can establish that the differences observed are due to treatments or techniques rather than confounding factors such as the age, gender or comorbidities of patients,” said van de Velde.
Nine independently founded, up and running, national colorectal audit registers have already committed to participating in EURECCA (see below).
 A track record of improvements
A track record of improvements
These established systems offer an insight into the enormous gains that can be achieved through audit. Take the example of Denmark – after the introduction of a national colorectal cancer database in 1994, the five-year overall survival for rectal cancer rose from 37% of patients in 1994 to 51% in 2006. A similar story emerges with the Norwegian colorectal cancer registry, where five-year survival for colorectal cancer patients increased after its launch in 1993, from 55% to 71%.
“Established systems offer an insight into the enormous gains that can be achieved through audit”
If EURECCA is to succeed in its aim to roll out the audit framework to address other solid tumour types, additional registries will need to be developed for instance in breast, oesophageal and gastric cancers. The framework will be multidisciplinary, including information not just on surgery but also on medical and radiation oncology. In addition to showing overall survival statistics, the system could also flag up specific complications of surgery or side-effects associated with drug treatments. “Cancer is like a war situation – not only do many people die from the disease, but survivors experience enormous suffering. All these data could be represented in the analysis, creating the possibility for identifying strategies that prevent problems,” says van de Velde.
Patient’s views on treatments and the approach of health staff may also eventually be incorporated into the mix. “The system could identify reasons why patients perceive one centre to be better than other, and indicate changes that could be implemented to make services more user friendly.”
EURECCA offers the potential to transform the development of cancer guidelines and allow them to represent ‘real world’ patient situations. “Traditionally guidelines have been based on the beliefs of charismatic leaders backed up by clinical trials,” explains van de Velde. “Incorporating the EURECCA data would allow guidelines to become much more evidence based and include data on patients who are normally excluded from clinical trials, either because they’re too old or have comorbidities.”
Ultimately, he adds, the hope is that EURECCA will offer an online system giving individual cancer centres access to their own confidential data, which would also allow them to compare their performance with other anonymised centres across Europe. “The approach is like giving health professionals a mirror. It allows them to take a good look at themselves and identify how things might be improved.”
Where centres are found to be underperforming, mechanisms will need to be introduced to help improve practice, and also identify health professionals who need retraining. But key to the success of the initiative, van de Velde insists, is that there should be no public naming, blaming and shaming of individuals or institutions.
A patient’s right to know
The EURECCA initiative was given a very enthusiastic reception at the meeting, but many felt that clinicians and hospitals do need to be held accountable for performances.
“With people’s lives at stake, professional sensitivities need to be put to one side,” said Redmond. If patients are to actively participate in the decision-making process, added Regine Hagmann, from the German Cancer Information Service, there is a need for full transparency. How can they make informed decisions about where to go and who to trust if outcome information is all anonymised? Survival statistics are the ones patients need most of all, commented Stella Kyriakides, chair of the ECCO Patient Advisory Committee, and these should be publicly available.
“If patients are to participate in the decision-making process, there is a need for full transparency”
Redmond offered an example from Italy to show that making quality-related information publicly available does help pull up standards. A leading Italian daily paper, the Corriere della Sera now publishes data for every hospital in the country, showing the number of cancer patients that they treat annually, broken down by cancer type. The figures are collected by the health authorities, but it is the newspaper that presents them on their own website (www.corriere.it/salute/sportello_cancro/), in a way that is easy to search – you just click the relevant body part to identify the cancer of interest, and then click a region on a map of Italy for details on every hospital in the region. The initiative led to patients identifying the centres that performed only a few procedures annually and ‘voting with their feet’ to avoid them.
The natural competitive instincts of countries, healthcare regions and individual clinicians can all be leveraged to the patient’s advantage, said Kerr. When the EUROCARE survey identified the UK as a country with poor national cancer survival figures, it shamed the former Prime Minister, Tony Blair, into launching the National Cancer Plan.
In the UK the process could soon be taken one step further. The White Paper ‘Equity and Excellence: Liberating the NHS,’ published in October 2010 by the Department of Health, is looking to create a system that will allow clinical teams to see meaningful, risk-adjusted assessments of their performance against their peers. The Paper plans for the information to be placed in the public domain, allowing patients to check on the care results of individual doctors before choosing their provider.
Recording the five-year survival rates of individual clinicians, said Kerr, is likely to result in the creation of league tables in the UK. “The professional pride of clinicians finding themselves at the bottom of such tables would doubtless be responsible for improving care, which would drive up standards,” he said.





