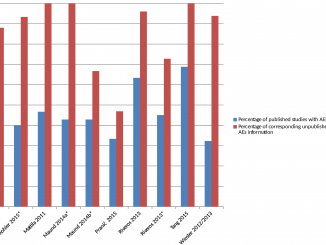
Adverse events appear to be systematically underreported in clinical trials
«There is strong evidence that much of the information on adverse events remains unpublished and that the number and range of adverse events is higher in unpublished than in published versions of the same study»: [more]