Brain tumours are tough to treat for so many reasons. Progress depends almost entirely on the steadfast commitment of doctors working within international networks. Peter McIntyre talked to some of them, to hear about their hopes, their frustrations, and what keeps them going.
It’s now 12 years since an editorial in the New England Journal of Medicine hailed “a new beginning” for chemotherapy in brain tumours, on the back of the trial that established temozolomide following radiotherapy as a new standard of care for glioblastoma multiforme – one of the most aggressive of all cancers (NEJM, 2005; 352:1036–38).
The optimism seemed justified at the time. Not only did this new cytotoxic increase median survival from 12 to 15 months – more than doubling the two-year survival rate from 11% to 27% – but an accompanying study even identified a biomarker – MGMT methylation – that predicts which patients will do much better than the median and which will do worse.
Yet it was a modest beginning, as the researchers well understood. By the time the five-year follow up report was published, confirming the initial findings, 93% of the patients on that trial had died (Lancet Oncol 2009, 10:459–466).
And while that new beginning has been followed by important advances in understanding brain cancers, progress finding new treatments has been frustratingly slow Twelve years on, the standard of care is still radiotherapy plus temozo-lomide.
Over the past decade a number of promising drugs – monoclonal antibodies and immunotherapies – have failed on clinical trials, either because they cannot cross the blood brain barrier, or because brain tumour cells are so diverse in their genetic and metabolic compositions.
“Whether it’s your cognitive or physical abilities,
every single part of who you are
can be affected by a brain tumour”
It can be dispiriting work, and yet the networks of specialists that were key to establishing the benefits of temozolomide remain as strong and determined as ever, driven by the continuing urgent need to find solutions for their patients.
Fruits of collaboration
Brain tumours are among the most deadly and difficult cancers to treat. While many people live with a low-grade glioma for 10 or 20 years, the majority of aggressive cancers return after surgery, and life expectancy can be measured in months.
As Kathy Oliver, co-founder and chair of the International Brain Tumour Alliance, explains, brain tumours touch all aspects of a patient’s life. “Whether it is your cognitive abilities or your physical abilities, every single part of who you are can be affected by a brain tumour, and your quality of life and that of the whole family can suffer enormously.”
As well as being hard to treat, brain tumours are also rare, which make them commercially unattractive. Progress continues to rely almost entirely on the unstinting efforts of specialists pooling their efforts in collaborative projects.
The clinical trial that established temozolomide as a standard of care was led from the University of Lausanne, Switzerland, by a young oncologist named Roger Stupp, who, on arriving fresh from qualifying as a haematologist/oncologist in the US, had found himself assigned to “what other people didn’t want to do”. It was sponsored by the European Organisation for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada Clinical Trials Group (NCIC), and it involved 85 institutes in Europe and Canada, recruiting 573 patients across 15 countries.
What really strikes home from this and subsequent research is the sheer number of centres collaborating across countries, and the length of time. Dozens of researchers have devoted whole careers to painstaking work and testing, enrolling thousands of patients from dozens of countries to make progress. The origins of a number of trials that are still running today date back before the start of this millennium. Such a long-term collaborative process can only be handled by organisations with international status and a core of clinical excellence.
The EORTC and its brain tumour and radiotherapy groups drive research ideas and plan collaboration across Europe. In Canada it is the Canadian Cancer Trials Group and the Canadian Brain Tumour Consortium. In the US many centres are affiliated to the NCI-funded NRG Oncology (formerly Radiation Therapy Oncology Group) or Alliance for Clinical Trials in Oncology, while the Trans-Tasman Radiation Oncology Group (TROG) has more than 1,000 members in Australia and New Zealand.
“A company will shift its focus;
we have to answer these real questions
on the best management of patients”
EORTC Director General Denis Lacombe says that linking independent-minded researchers in academic networks with a central organising body can make a real difference to patient care.
“If you look at the plenary session of ASCO, the vast majority of big studies that make a difference are academic studies. Neuro-oncology is an area where few of the drugs that have been tried have made headway. There is a need to work together to exchange ideas and do projects, because we are still in the learning phase of this disease. It is a group effort; a very good example of a large network identifying unmet need and having this continuity over time.”
Michael Weller, head of neurology at University Hospital Zurich and chair of the EORTC Brain Tumour Group, says that three headline presentations at ASCO 2016 will help establish new treatment protocols and improve survival and quality of life.
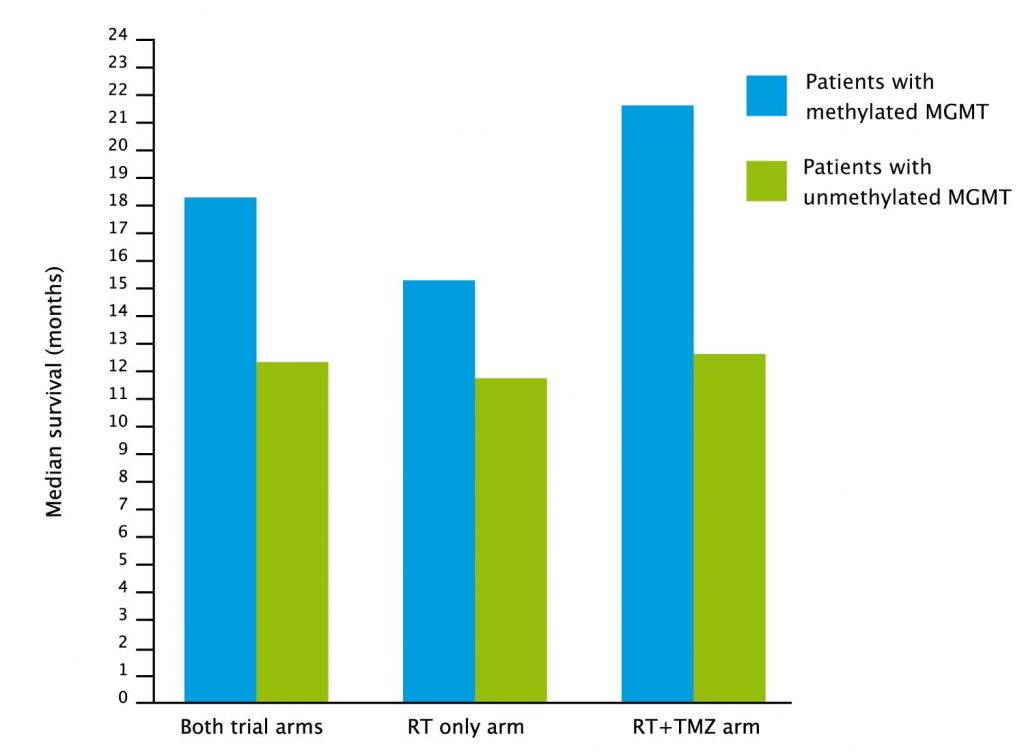
Temozolomide in older patients
The Canadian Cancer Trials Group, together with EORTC and TROG, trialled temozolomide in combination with radiotherapy in 562 patients with glioblastoma, with an average age of 73 – the first time this had been tried in a full phase III trial conducted in older patients.
They showed that adding temozolomide to a shorter course of radiation therapy improved survival without damaging quality of life. Two-year survival rose from 2.8% without temozolomide to 10.4% with combination treatment.
The benefit was greater for the 165 patients who had MGMT methylation.
Temozolomide in anaplastic glioma
A second presentation at ASCO 2016 focused on patients with anaplastic glioma. Some patients live many years with these tumours, but they are almost always fatal in the end.
Earlier trials had shown the benefit of chemotherapy following radiotherapy – either a combination of PCV (procarbazine, lomustine, and vincristine) or temozolomide. PCV benefits were primarily in patients with 1p/19q co-deletion, a genetic marker that seems to indicate greater sensitivity to chemotherapy.
The CATNON trial was established by EORTC to examine options for combining radiotherapy and temozolomide in anaplastic glioma patients who do not have 1p/19q co-deletion.
“Patients treated outside the trial – that is what I call failure.
Inside the trial I always learn something”
Because this is a relatively rare cancer and the primary endpoint is overall survival, this is another large and long trial, with 751 patients recruited from 132 centres in 12 countries in North America, Europe and Australia. The first patient was recruited in December 2007 and the trial will continue until 2020, with final results due in 2022.
Unexpected and welcome preliminary results led to the 2016 ASCO presentation. Study coordinator Martin van den Bent reported that patients treated with maintenance temozolomide following radiotherapy showed a significant increase in five-year survival, from 44% (without temozolomide) to 56% with it.
The CATNON presentation became one of the ten most read reports at ASCO, and the ASCO expert in brain cancers, Brian Alexander, welcomed the results and the long road to reach them. “For decades, anaplastic glioma has proven not only hard to treat, but also hard to study, because it is so rare, making this finding even more important.”
Like other leading members of the EORTC network, Martin van den Bent has been working to improve treatments for patients with brain tumours since the late 1990s. He says that collaboration between North American, Australian and European groups has been essential to a series of trials that has gradually established that chemotherapy plays a role in the management of nearly all diffuse gliomas.
“We understood that there was no way that any of the individual groups could successfully conclude the CATNON trial. A company will shift its focus; we are stuck in the marshes and have to answer these real questions that we have on the optimal management of patients. They are not driven by financial and economic considerations.”
However, van den Bent praises the commitment that the pharmaceutical company Schering Plough (now part of MSD) made to the trial, even though directors knew that its patent on temozolomide would expire before the CATNON trial concluded. “The clinical vision of the people at Schering Plough and their willingness to go beyond the classical business model has to be noted.”
Bevacizumab in glioblastoma
The third report at ASCO was disappointing. The monoclonal antibody bevacizumab showed promise in a phase II trial in patients whose glioblastoma showed progression. However, an EORTC phase III trial, led by Wolfgang Wick, past chair of the EORTC Brain Tumor Group, found that, while combining bevacizumab with chemotherapy improved progression-free survival, there was no overall survival advantage.
In it for the long term
Such disappointments are the backdrop to the search for progress. The integrin inhibitor cilengitide seemed a highly promising agent that could disrupt communication between glioblastoma cells and the brain microenvironment, until an international phase III trial, led by Roger Stupp, reported in 2014 that it brought no extra benefit added to chemotherapy. The trial had the backing of EORTC, the Canadian Brain Tumour Consortium, and the CENTRIC study team, and it included more than 500 patients with glioblastoma from 146 study sites in 25 countries.
Stupp, who is President of the EORTC, says that even disappointing trials should not be thought of as failures, since they help to prevent patients being exposed to potentially toxic expensive treatment with limited benefit, while the outcome data and tissue samples have the potential to improve understanding of the disease and develop other therapeutic targets.
“When you conduct a scientific experiment, the result can be ‘yes’ or ‘no’. If I get a clear result that something is not working, I don’t have the outcome I want, but the experiment worked. From the science point of view, failure means not recruiting or conducting the trial successfully. Patients treated outside the trial – that is what I call failure. Inside the trial, I always learn something for the next generation while giving the best available treatments to my patients.”
Brigitta Baumert was principal investigator on another large EORTC trial comparing temozolomide with radiotherapy in 477 patients with a high-risk low-grade glioma.
These are generally the slowest growing gliomas in adults – some people live with them for 10 or 20 years – but risk factors for more aggressive growth include age, tumour size and position, and the presence of neurological symptoms or epileptic seizures.
Baumert points out that the idea for this study was born in 1999 and it was the first study in brain tumours to mandate central molecular tumour characterisation before the inclusion of patients. “From having the idea, to getting the approval, and getting the core group set up took about four years. It took more years to get ethical approval from all national and international committees. Only after that can you run the trial.”

Patients started treatment between 2005 and 2012, and because of the long median-survival times, the primary endpoint was progression-free survival. with correlative analyses of progression-free survival by molecular markers as one of the secondary endpoints. In all, 78 participating centres from 19 countries were required to achieve the necessary patient numbers. Close collaboration with translational scientists like Monika Hegi, who is spearheading this effort within EORTC, is a key characteristic of academic research of this sort, which is ultimately designed to tailor treatment to a patient’s individual risk profile.
After four years of follow up, there was no difference in progression-free survival between the two treatment strategies. A further 5–10 years are needed to assess overall survival and cognitive effects of the two treatments and to identify any genetic groupings. Baumert believed it was essential to publish the results. However, it is harder to publish negative than positive results, and it was two years before the trial report appeared in Lancet Oncology.
Since her research began in Maastricht all those years ago, Baumert has moved jobs twice. But in this model of research, clinicians stay with their project even when they move. Indeed Roger Stupp is himself shortly leaving Switzerland to take up a new post in Chicago, at the Northwestern University’s Brain Tumor Institute. “For such international cooperation you need a very long breath,” says Baumert.
The value of independent research
Denis Lacombe, Director General of the EORTC, highlights the importance of research that is free from commercial interests. “Since we started these trials 15 years ago, this has led to the greatest therapeutic improvement for grade 4 glioma patients and we have learned a lot about the biology. The disease is still very aggressive, but we are moving from a completely deadly disease to one where therapeutic progress is being made.
“We have collected biological material from these patients, and this is not going to sit in a commercial silo just because a trial is negative. The material will be exploited to see what we can do more. The material collected from neuro-oncology patients is very precious. You have very small pieces and its use is discussed among a panel of experts. They are very cautious about the use of the material and the right question to ask next.”
Members of the EORTC Brain Tumour Group and Radiation Oncology Group met with other specialist EORTC groups for two days in early March 2017 in Brussels, to focus on immunotherapy, translational research and real-life effectiveness. Michael Weller sees this as an opportunity for cross-fertilisation. If a vaccine is effective for patients with one cancer, might it also be effective in other cancers with similar molecular markers? It would transform the prospects for treatment of glioblastoma if they could find a vaccine that could make a survival difference for 20–30% of the patient population.
However, Weller believes that academic groups need more financial support to continue to drive innovation. “EORTC landmark contributions have been ground shaking, because they change standards of care. But there is no way that academic institutions can face these challenges in terms of economic burden to do such trials in future. This is what I see as the major threat to how we can continue our successful work and attract companies to invest in it.
“The willingness of industry to invest in big trials is diminishing, especially if we pursue our strategy of identifying molecular subgroups. We are trying to dissect the diseases by their molecular markers, and this automatically makes them less prevalent in the population and a little less rewarding for a company to invest in. If we pursue our academic goal of individualising treatment, it is more difficult to find commercial partners.”
Working with the industry and patient advocates
Kathy Oliver, of the International Brain Tumour Alliance, believes that greater collaboration between research groups and industry, as well as greater involvement of patients and their advocates, will be essential for future progress. “There are fantastic people working in academic centres, but I think to really strike a home run, everyone is going to have to develop new models of working together across different stakeholder groups. Particularly for small patient populations as in brain tumours, it has to be everyone working together, including academia, industry and patients – the total deal.
“Part of the problem with a brain tumour is that it is such a difficult, tough disease to crack. You have to get the therapy across the blood brain barrier and that is a major hurdle. The incredibly challenging nature of treating a brain tumour may be one of the reasons why we are seeing very long clinical trials. Of course, patients are desperate to see research speeded up.”
Kathy Oliver, who lost her own son, Colin, to brain cancer, is a patient representative on the EORTC’s SISAQOL initiative to set international standards for analysing patient reported outcomes and quality of life data. She sees this as a very welcome step towards a level playing field. “I am welcome to have an equal voice to anyone else on that committee, which involves people from the FDA [US regulators], the MHRA/EMA [UK and European regulators], high-level clinicians, industry and leading researchers.”
“The biological material from these patients
is not going to sit in a commercial silo
just because a trial is negative”
It is important for clinical trial designers and researchers to listen more carefully to what patients want out of clinical trials and out of therapies, she says.
“Word of a good clinical trial spreads like wildfire through the patient community. If patients think that a trial is good and important and useful, then they will tell other patients. So I think that is one practical way of speeding up trials. In my opinion, trials will recruit faster if patients are involved in helping to design them.”
Other approaches
Of course EORTC and their partners on other continents are not the only game in the global town. The GBM Agile trial (see page 61) – principally an Australian/US initiative, now also involving China, is putting together a trial infrastructure that can test a variety of treatments against the standard of care for glioblastoma, across different molecular subgroups of patients. EORTC has itself established a screening infrastructure, SPECTAbrain, to channel patients with brain tumours into relevant biomarker-driven trials, with samples being held in their biobank. The aim is also to speed up the investigation for biomarkers and develop high-quality testing standards for those markers.
SPECTAbrain is open for business, thanks in part to initial support from Celldex, but it will need more buy-in from pharmaceutical companies. “We like SPECTAbrain, but it needs to be financed,” says Weller. “We need to generate more revenue and actionable targets to keep it alive.”
There is also a non-drug treatment that is making waves. The FDA has approved for adjuvant use a device that is worn by patients and delivers low-intensity, intermediate-frequency, alternating electric fields directly on the scalp through electrodes. This Optune device may sound as daft as crystal treatment, but benefits have been shown by a large randomised clinical trial led by Roger Stupp, which demonstrated longer progression-free survival and overall survival.
Roger Stupp says the Tumor Treating Fields (Optune) results provide a lesson in following the data rather than preconceived notions. “It is a nice example of how something which to many of us looks like voodoo medicine has shown it improves survival in a similar magnitude to temozolomide ten years earlier.”
“Trials will recruit faster if patients are involved
in helping to design them”
He contrasts this with the results from some of the newer drugs at the cutting edge of knowledge. “We have been jumping up and down about immunotherapy for 25 years and so far [in brain tumours], nothing works.”
The future
Stupp says there is no short cut to finding better treatments – it requires patience and systematic work. “If you ask me to predict what will succeed, then I am going to be very inaccurate because we don’t really know. Let’s be honest. When we had successes it was not really predicted.
“The advances came when we really sat down and did things systematically and one after the other and put the resources together rather than everybody on his own.”
His biggest frustration is that nothing has yet replaced temozolomide. However, standards of care for people with brain tumours have continued to improve. “If you look at what really has happened since temozolomide became available, there is more awareness. Before, patients got radiation and steroids and were sent to hospice care. Now we have a better delivery of care for patients with brain tumours, independent of chemotherapy.”
He expects that molecular signatures will eventually identify patients most likely to benefit from new treatments, even though many molecular markers, such as EGFR amplification and IDH, contribute little to daily decision making in the choice of therapeutic agent so far. “It is disappointing we have only 10–15% of patients alive at five years, but it is particularly disappointing we have not been able to identify which 15%. A kind of ‘one fits all’ approach is probably one reason we are not that successful.
“If 60–70% of patients in a clinical trial just produce noise, you may miss the true signal that tells you that an agent and an avenue may be active. There may be good avenues, good ideas and good treatments that we have discarded because we have not been able to recognise the activity.”
In recent years, dozens of clinical trials on glioblastoma treatments that have shown preclinical promise have shown minimal quality of life or overall survival benefit. Many were stopped early. Rindopepimut is one immunotherapy agent that looked highly promising in uncontrolled phase II trials in patients with newly diagnosed EGFR-positive glioblastoma. An industry-sponsored international phase III study, with substantial contributions from EORTC researchers led by Weller, was discontinued in March 2016 when it became clear that the vaccine did not improve survival beyond standard care.
But as activated EGFR is found in more than 40% of all glioblastomas, no one is giving up looking. EORTC and the global pharmaceutical company AbbVie are awaiting results from a recently completed trial with ABT 414, an antibody-drug conjugate that binds to an EGFR epitope. This trial, involving 240 patients in 22 countries, is another example of collaboration between the EORTC and a commercial company.
“The advances came when we did things systematically,
one after the other, and put the resources together”
One critical point will be to ensure that innovative agents do indeed reach their targets. “Some of the EGFR agents that have been tested do not cross the blood brain barrier. These are agents that, by their chemical properties, are not the right agents to study in brain tumours,” says Stupp.
Some glioblastoma patients treated in the late 1990s are still alive 20 years later, and one promising line of research is to identify patients who defy the odds. The Brain Tumor Funders Collaborative (BTFC) of north America has put $2 million into an EORTC-led study to understand the reasons for such long-term survival. This research, headed by Michael Weller, will study more than 300 patients who have survived glioblastoma for more than five years, and will involve analysing tumour biopsies banked by the EORTC and other academic groups over many years.
“Some say in 10 years immunotherapy
may have replaced chemotherapy,
but this is no excuse not to continue”
Martin van den Bent believes there is still some room for improvement in combinations of existing treat-ments. “We have established that chemotherapy works and the basic questions for diffuse glioma in chemotherapy have been answered.
“What we have not really answered is the question on the optimal timing of chemotherapy treatment in low-grade glioma. It will be a difficult project and it will take 10 to 20 years to complete, but this is about whether we can postpone safely treatment in patients and avoid side effects or can we improve outcomes. Quality of life will be an important question.”
Some may say that in ten years immunotherapy will have replaced chemotherapy, but van den Bent says this is no excuse not to continue. “Over the past decade, when we were making these efforts, people said we will have new drugs. We are now ten years down the line, the drug is not on the horizon and we are glad we made the effort.
“Of course I would love to see the breakthrough drug. However, the indications are that diffuse gliomas show too much variance and that one drug is likely to affect only a limited proportion of patients.”
Weller on the other hand is much more optimistic about immunotherapy. “I am sure that what we can do with radiation and chemotherapy is done.
I am very optimistic we are going to see some progress in immunotherapy, and probably also some novel concepts stopping the invasion of tumour cells. It is about understanding how we can make tumour cells identifiable by the immune system, and understanding what is different biochemically and metabolically and then going selectively after the tumour. That is all part of individualising cancer therapy.”
Who will pay?
If the road ahead looks long and uncertain, how will research be funded?
The EORTC Cancer Research Fund is supported by some national cancer leagues, social responsibility programmes and charitable donations. Many trials are partly funded by foundations; support that is vital since answers to many of these questions have no direct commercial benefit and will not be supported by industry.
Stupp is convinced that cooperation with pharmaceutical companies, and conduct of carefully designed clinical and translational research by an independent organisation like EORTC, is the most efficient way to benefit patients.
Academia contributes in kind substantially to clinical research, with hundreds of hours of clinician and research associate input, without financial benefit, even if one of the trials is spectacularly successful.
“Our patients do not want to give up,
and I get energy from my patients”
While EORTC can bid for grants from the EU Horizon 2020 funding, there is no direct funding from the EU. Denis Lacombe says: “In an ideal world, the EU would recognise the EORTC as the clinical research infrastructure at a European level and give some core support. I think it is a dream that will never happen. We have some European money based on a competitive approach, but absolutely no core European money. Absolutely not.”
Nobody likes to fund the growing infrastructure requirements of clinical research. Stupp says that the benefits of expertise, quality assurance, innovation and dedication are seriously undervalued, as they spread beyond research to patients in routine clinical care. “If we are not careful, we are going to suffocate the system,” he warns.
So what makes a clinician stay with a line of research for decades knowing that disappointment is as likely as success? Roger Stupp says that oncologists need be able to tolerate some frustration because the disease is so difficult.
“Our patients do not want to give up, and I get energy from my patients. As a researcher you need curiosity and openness and rigour in order to test something in a scientific way.”
EORTC has a 53-year record of working for improvements in patient care, and Lacombe says that this will continue. “We have a commitment to patients. If we think that a research question is important for patients, we make it happen. We say to our scientists and our doctors, EORTC is the place to go because we have the capacity to do this kind of international trial. If you have a good idea and it is a good project, we will find a way.”