Can deep and close-knit collaboration between a handful of elite centres achieve what broader European research platforms and projects cannot?
 There has been talk about setting up a European cancer institute to rival the American National Cancer Institute (NCI) and its network of comprehensive cancer centres for many years. No such large-scale driver of research has ever been established, however, either on the NCI model or any alternative.
There has been talk about setting up a European cancer institute to rival the American National Cancer Institute (NCI) and its network of comprehensive cancer centres for many years. No such large-scale driver of research has ever been established, however, either on the NCI model or any alternative.
Instead, we have seen a plethora of European networking projects that typically have limited lifespans, such as those funded by the European Commission’s framework programmes, and several organisations that can lay claim to much good pan-European collaborative work, in particular the European Organisation for the Research and Treatment of Cancer. Founded in the 1960s – more than 20 years after the NCI – the EORTC is the closest Europe has come to an institute model, but it is far smaller in terms of funding and has no laboratories of its own.
In any case, the vast majority of cancer research funding is spent within countries, and probably the biggest research collaborations are those such as the UK’s Experimental Cancer Medicine Centre (ECMC) initiative and Cancer Research UK (CRUK) projects, France’s Cancéropôle networks and the German Cancer Consortium (DKTK), which include a wide range of basic and clinical research.
A key characteristic of some of these national initiatives is that they tend to select ‘core’ centres with the best expertise – CRUK, for example, provides a substantial proportion of its scientific funding to just five research institutes, including Cambridge, Oxford and Manchester. Most collaborative networks involve many centres with interests in certain topics, such as leukaemias and childhood cancers, but ‘core’ implies a depth of expertise where institutes can contribute fundamental parts of translational cancer research to make a more joined up ‘whole’.
Certainly, funders and researchers are recognising that cancer research has long reached a scale and complexity that is beyond any single institute, and many of the most challenging questions now require joint working among partners with equal standing in the depth of their contributions.
About ten years ago EORTC aimed to establish its Network of Core Institutes (NOCI) – around 20 European centres that have the patient numbers and multidisciplinary groups needed to participate in increasingly complex translational research studies. Meanwhile, the EurocanPlatform from the European Commission has also been active in establishing a concept for translational research in Europe, involving more than 20 cancer centres, some of which also committed to the virtual institute idea in 2008 with the Stockholm Declaration, masterminded by Ulrik Ringborg at the Karolinska. Ringborg has also been instrumental in the work of the Organisation of European Cancer Institutes (OECI), which includes research excellence in its accreditation scheme.
It has been hard, however, to establish major trial work at this level. Martine Piccart, a past EORTC president, reported at the organisation’s 50th anniversary conference that it had been much more difficult than anticipated to get NOCI up and running, because it needs a ‘horizontal’ platform among centres, for information integration, biomarker testing, biopsies, sample logistics and much more.
 By 2012, only a few such trials were underway, although the often discussed MINDACT trial, which aimed to validate a gene signature to guide treatment in early breast cancer, has been the vanguard. There was seven years of intensive collaborative work and a struggle to get funding, noted Piccart. A European Union grant of €7 million fell far short of the actual cost of around €45 million to recruit 6,600 women across Europe for the work. But it was a big step in the development of translational research across Europe, and was the first such oncology trial to be supported by an EU grant (from the research framework programme).
By 2012, only a few such trials were underway, although the often discussed MINDACT trial, which aimed to validate a gene signature to guide treatment in early breast cancer, has been the vanguard. There was seven years of intensive collaborative work and a struggle to get funding, noted Piccart. A European Union grant of €7 million fell far short of the actual cost of around €45 million to recruit 6,600 women across Europe for the work. But it was a big step in the development of translational research across Europe, and was the first such oncology trial to be supported by an EU grant (from the research framework programme).
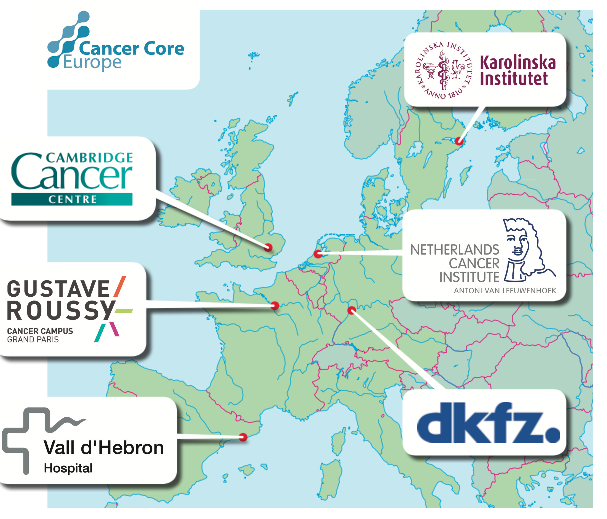
It was under Lex Eggermont’s presidency of EORTC that the NOCI idea came about. Now he and colleagues at six of the major cancer and research centres in Europe, which are also active in the EurocanPlatform, have set up an elite group of core institutes to try to cut through the many obstacles that have slowed translational research. Eggermont’s own centre, Gustave Roussy in Paris, where he is director, has formed Cancer Core Europe, with the Cambridge Cancer Centre, the Karolinska in Stockholm, the Netherlands Cancer Institute in Amsterdam, Vall d’Hebron in Barcelona, and the National Centre for Tumour Diseases (DKFZ) in Heidelberg, Germany.
There is no doubt these are top centres, and hold a balance between clinical and basic research, and they have worked together anyway in various consortiums. But as Eggermont points out: “The problem with a lot of consortiums is that they are often constructed around a project model, such as the EU framework programmes, and there are just too many partners and work packages – one tries to do everything. It is very difficult to create sustainable activity. We want to create a network built on infrastructure that will last, starting with a few centres that know each other well and are committed to it.”
A critical mass
The aim, he adds, is to take advantage of the scale and expertise of the six institutes to establish a critical mass for a prospective dataset that is clinically annotated and is a far better platform for researching precision medicine and personalised treatment.
“We have 60,000 newly diagnosed cases each year among the six centres, treat 300,000 patients and follow up a further 1.4 million. We need to unite them in a prospective way, because retrospective databases are like Swiss cheese – they are full of holes that are extremely laborious to fill because oncology is just too complex.”
Eggermont and partners in Cancer Core Europe are calling the initiative a virtual ‘e-hospital’ with the emphasis on data sharing and compatibility for processes such as molecular profiling and standard operating procedures such as tissue processing. “Precision medicine needs to be understood as one big project and we need to put an end to fragmented data warehouses,” he says. Five years ago, cancer institutes thought they could “go it alone”, he adds, but with the exception of the Sanger Institute, in Cambridge, which has world-class genomic expertise, none have the data to work on the complex questions.
“Precision medicine needs to be one big project and we need to put an end to fragmented data warehouses”
He places great emphasis on what he calls “harmonising readout understanding”, meaning say the outputs from biomarker assays and gene profiling, so that close-knit groups of researchers across the institutes are working to the same rules. Simply scaling up data without harmonisation will just amplify lack of quality, and make it far more difficult to reach the goal of more rapid and reliable outcome research, he says.
Cancer Core Europe has appointed a scientific officer to develop its work programme. Fabien Calvo is a pharmacology professor, a past deputy at France’s National Cancer Institute (INCa) and has been part of numerous research networks, including co-launching the International Cancer Genome Consortium in 2008. “I can see from travelling among the six sites that there is outstanding research at each but also some weaknesses, but they are really good representatives of what can be done at national level,” says Calvo. The institutes are genuinely also world leaders, he adds, but have particular strengths, such as proteo-mics at the Karolinska and early-phase trials at Gustave Roussy.
He confirms that establishing data sharing infrastructure is the first step – and also probably the most complicated. “Then we want to coordinate clinical trials, especially for targeted medicines, and we are working with pharma to share information and we need them to provide drugs.” Also on the work programme is molecular analysis of tumours and the development of biomarkers.
That some of the institutes were collaborating before the official Cancer Core Europe announcement was confirmed by Carlos Caldas, professor of cancer medicine at Cambridge, speaking at the launch last year. He noted that a breast cancer trial was about to be started by Cambridge, Vall d’Hebron and the Netherlands Cancer Institute, with drug firm Genentech. “I don’t think this trial would have been possible at any of the institutions individually,” he said. It is a “very demanding” trial using the latest imaging and profiling of circulating tumour DNA and will also involve Gustave Roussy and maybe the other Cancer Core Europe institutes. It’s a true investigator- and science-led trial, rather than a typical pharma-led trial, where a company spreads its own protocol among a wide number of centres, he added.
“It’s a very demanding, science-led trial that would not have been possible at any of the institutions individually”
But funding from pharma is clearly important, and Eggermont says that Cancer Core Europe will also look for grants from various sources. He notes that the EU’s EIT Health/InnoLife initiative on healthy living and active aging has selected Cancer Core Europe to represent oncology in current bid preparation. “They like the infrastructure we are building,” he says.
World leading collaboration
That infrastructure could also be world leading, given that, despite the dollars flowing into US research, the American cancer centres are not actually networked in this way, although some are very large. “The main institutes in the US are all islands,” says Eggermont. The US is good at joint genomic research, adds Calvo, but not so good at clinical trials, where collaboration matters for translational research, and the cancer community is not as well funded as might be thought.
President Barack Obama has recently called for a drive for precision medicine, but Harold Varmus, the Nobel laureate who has recently stepped down as director of the NCI, has spoken out about a “shocking” drop in the institute’s budget. He said that basic research is the fuel for innovation – advances such as immunotherapies are testimony to this – but there is so much more to be done, and he stressed that Americans should not become “slackers” in “funding the most fundamental things”.
Eggermont agrees about the importance of basic research but adds that the Cancer Core Europe concept is also fundamental to organising the “chaos” that bringing discoveries out of the lab to the clinic can bring.
Chaos could also be a good word to apply to the state of European cancer research, a topic that Richard Sullivan, director of the Institute of Cancer Policy at King’s Health Partners Integrated Cancer Centre in London, knows only too well. Writing back in 2008 on the possibilities for a European institute, he was prescient in saying that the big cancer centres were likely to take the lead on their own, given the lack of direction and funding from national and European organisations.
He points out though that it may be premature to launch Cancer Core Europe, as the EurocanPlatform is still under analysis as a model, and that the six institutes are by no means the only major research institutes in Europe. “It does raise the question about how we get the best out of the best researchers,” he says. “Of course they are powerful centres with lots of great technology, but what really is going to happen that wouldn’t happen otherwise, because there are lots of other collaborations in this crowded translational space, such as the WIN Consortium, also led by Gustave Roussy, the EORTC, and the Breast International Group, to name but a few.”
Undoubtedly there are strong national networks too – the UK’s Experimental Cancer Medicine Centre initiative, for example, looks similar to Cancer Core Europe, as it reports that over the last seven years it has supported more than 1,000 early-phase trials and 700 biomarker studies, and aims to harmonise trials. Other European projects include Cancer-ID, a public–private consortium supported by Europe’s Innovative Medicines Initiative that is validating blood-based biomarkers. Eggermont’s response is again about sustainability, level of expertise and whether the goals really are similar.
It’s also the case, says Sullivan, that cancer research is much bigger than the translational platform that Cancer Core Europe is pursuing. “What benefits patients is also research into surgery, radiotherapy and palliative care to name but a few areas.”
“We also need a view on whether there is a strategic plan and how we can measure success in say five years’ time,” he adds, “and I also have a question about how inclusive or exclusive this initiative will be. Will it truly capture the best Europe has to offer?”
Sullivan says that the EU’s Horizon 2020 research programme should spread its net wide to capture innovation across the continent. “There are smaller institutes doing niche work in areas such as imaging, and countries like Poland are producing some excellent work. Because cancer has become such an important area of biomedical research, a lot of governments and charities are now investing heavily in it. We tend to think that Europe is smaller than North America but really it isn’t in terms of impact in cancer research.”
Eggermont stresses that Cancer Core Europe will open the door to other centres in Europe, although the bar to join will be set “very high”. Soon there will be a website and work programme to view, and the cancer community can start to decide whether there is a new powerhouse that will take everyone forward.






Leave a Reply