
The European Commission is developing a Europe-wide accreditation scheme for breast centres to push up standards of diagnosis and care. Here experts from both sides of the Atlantic take a look at existing schemes, the criteria they use and the challenges in applying them across diverse populations.
 Cary Kaufman: In the US we were prompted to change our approach to breast cancer care in response to a number of factors, including two reports from the Institute of Medicine (1999, 2013) demonstrating that many patients did not receive the care they should. We wanted to reduce the wide gap between the care that many breast cancer patients experienced and the ideal treatment they should be receiving. We also wanted to improve the value of healthcare by increasing the quality while possibly decreasing the cost, with these two factors going hand in hand.
Cary Kaufman: In the US we were prompted to change our approach to breast cancer care in response to a number of factors, including two reports from the Institute of Medicine (1999, 2013) demonstrating that many patients did not receive the care they should. We wanted to reduce the wide gap between the care that many breast cancer patients experienced and the ideal treatment they should be receiving. We also wanted to improve the value of healthcare by increasing the quality while possibly decreasing the cost, with these two factors going hand in hand.
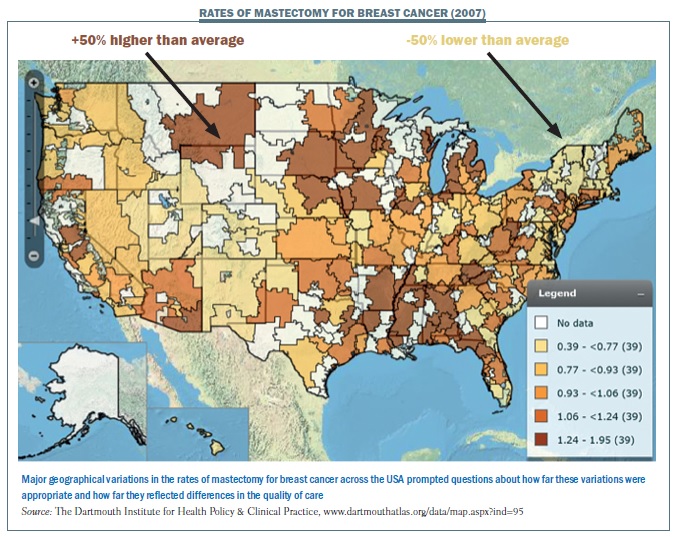
A map of the US (see below) shows the wide range in use of mastectomy in 2007, with some regions having a 50% higher than average use of mastectomy (shown in dark brown) while others had a 50% lower use than average (shown in light tan). Why was that? Some areas may have had appropriate rates, but we wanted to know whether mastectomy was being used too much or too little for individual patients. We wanted to be sure that it is being used appropriately. Maps for use of radiation therapy, systemic chemotherapy or breast reconstruction would show the same type of mosaic, and we need to be sure that the kind of care that people should get is what they actually do get.
 The National Accreditation Program for Breast Centers (NAPBC) was set up in 2005 to address three main issues:
The National Accreditation Program for Breast Centers (NAPBC) was set up in 2005 to address three main issues:
- gaps between the desired care and the actual care that women were receiving
- the need for adequate written guidelines to impose consistency of breast care
- the recognition that standards should be written by the clinicians and not by the payers or government.
We invited 21 organisations encompassing the range of professionals involved in breast cancer care, including the American College of Surgeons, the American Society of Clinical Oncology and the American Society for Radiation Oncology, to join with us to figure out what standards should be provided by a specialised breast unit or breast centre.
We divided into five committees, organised to identify key concepts that were passed on to other committees to develop further:
- Quality – to identify key quality breast cancer care concepts, such as recommending needle biopsy rather than surgical biopsy
- Standards – to develop and write standards for quality concepts that are universally applicable across different breast cancer centres
- Education – to disseminate standards to providers
- Advocacy – to disseminate standards to patients and the public
- International – to disseminate and collaborate outside the US, to be sure that we are all asking the same questions and can learn from one another.
Accreditation process
It generally takes six to nine months for the accreditation process, from the time a centre first looks at this until they receive a survey. This is not because we’re slow in sending out surveys, but because centres realise that they may not be providing the standards we are asking for. They may be providing high-quality care, but elements may be missing even at academic centres, for example the integration of care, communication between specialists, consideration of neoadjuvant chemotherapy for surgery, or holding a conference to discuss patients.
We start with an application process, where the centre applies, reads the requirements and then reviews the standards. They can upload documents to the survey application record (SAR), which is a computerised site where applicants can upload information. Once they have completed the data, the surveyor reviews the SAR. At that point we identify issues that need to be addressed and completed, so communication goes back and forth. Before any survey is carried out there is a lot of communication and upgrading of care to ensure that facilities comply with our standards.
Finally, a single surveyor goes out to the centre, already aware of the kind of care they are providing. The surveyor spends a day meeting with clinicians, attending meetings and multidisciplinary conferences, and looking at information including reviewing charts and discussing findings for both cancer and benign disease, recognising that breast centres take care of both.
The surveyor then makes their report and presents it to the site, and reports back to the centre on their findings, including advice on where they can improve – this may include things that are not on our standards if they find areas where the centre can improve. If the centre passes at least 90% of our 27 standards (24 out of 27) they are deemed accredited or certified. However, they must comply with 100% of the standards within one year.
Our Breast Cancer Center Standards Manual provides information on our standards, which are updated every year.
The manual has six chapters:
- Breast centre leadership
- Clinical management, which addresses physicians and allied healthcare disciplines
- Research, which we consider important and we require a certain number of patients participating in research at each centre
- Community outreach, including ensuring provision of screening and diagnosis
- Professional education, to maintain skills
- Quality improvement, to ensure centres comply with our quality improvement items. They also need to have at least two quality improvement projects each year that are focused on their own needs.
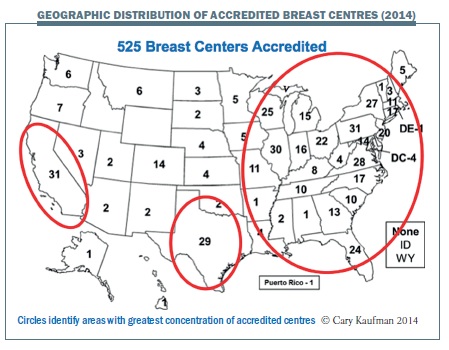
The figure below shows the geographic distribution of accredited breast centres across the US, with 525 breast centres currently accredited. Our state, Washington, has six centres that are accredited, which could be higher. The circles show the population concentration, which is where most centres are found.
 What are the standards?
What are the standards?
The standards can be divided into two main areas: administrative leadership and comprehensive clinical breast care. The administrative leadership is responsible, and should be independent and really focused on the breast centre. It should ensure that treatment guidelines are available for clinicians and are being followed, and confirm that providers are being educated and that quality programmes are being performed. The leadership should make sure that data for each patient is collected in a database so that the quality of care can be reviewed, and that the centre is participating in research and maintaining outreach to the community.
A comprehensive approach to clinical breast care should ensure that the ideal care is the actual care provided. There are three main areas:
- Interdisciplinary breast conference (or multidisciplinary meeting), where all clinicians meet to discuss a patient, including presentation of their case, data, mammograms and pathology. The team discusses what is the best approach for that individual patient, including any potentially appropriate research studies, optimising their integration and collaboration across all disciplines, with input from the most junior nurse to the most senior doctor.
- Clinical breast care, providing multidisciplinary care by specialised breast physicians across the entire range of disciplines.
- Allied breast care, which is the allied healthcare by multiprofessional providers that really makes a breast centre. Even the very best surgeon or radiation oncologist needs the glue that puts things together, with oncology nursing, patient navigation, genetics, research co-ordination, social workers, psychotherapy, physical therapy and survivorship.
Why do centres seek accreditation?
We asked 525 centres about their reasons for applying for voluntary accreditation and got 219 responses, with the main reason being to validate their high-quality breast cancer care (89%). Other reasons were executive leadership decisions, marketing and access to a national database, but the main reason is because centres want to improve their quality by complying with standards that are recognised by specialists.
Early on, medical university centres and National Cancer Institute (NCI) centres did not sign up, but it just took them longer. Today university centres account for 13% of all breast centres, and 28% of NCI-designated cancer centres are now NAPBC accredited.
Lastly, it is worth commenting on the difficulty in setting these standards and how we go about it. We have a standard that says the breast conservation rate should be at least 50%. On average in our centres the breast conservation rate is 66%, but some centres are below 50%, because women want a mastectomy and they have access to high-quality reconstructive procedures. On the other hand, some areas, such as Massachusetts, have a very low mastectomy rate, so I think when we set quality targets we have to adjust to the realities of location.
The European perspective
Fatima Cardoso: One of the challenges in Europe is that we have many different countries with different healthcare systems, regulatory systems and reimbursement systems. This leads to different access to care and access to different types of care, which impacts on the quality of care. This non-uniform situation is an extra hurdle for establishing a European accreditation or certification system. Some countries are more advanced than others, and have already developed their own national accreditation systems – Germany and Switzerland for example. However, they have different criteria, so when we try to do something at a European level we need to take existing national systems into account.
The European Society of Breast Cancer Specialists (EUSOMA) is leading their programme in Europe, and has developed a voluntary, uniform accreditation system that can be applied in any European country. However, it does not take into account the different realities in different countries. Mastectomy with immediate reconstruction is sometimes a better option than breast conserving surgery, where oncoplastic surgery is available. But high rates of mastectomy in countries where oncoplastic surgery is not available indicates inappropriate treatment. The availability of radiotherapy equipment is also important. However, there are quality criteria that are essential no matter where a breast centre is located. The system is voluntary, as in the USA, and there are pros and cons for making it mandatory. The European Commission is starting to develop a guidelines and accreditation project to be carried out at the European level, which could be a good way to go.
Realities differ across countries, but there are quality criteria that are essential wherever the centre is located The EUSOMA accreditation system was launched in 2002 and updated in 2007 (EJC 2007; 43:660–675). Certification is provided by an independent body, through the European Cancer Care Certification, and not by EUSOMA.
The most important criteria for a breast unit or centre are:
- A single integrated unit – as mentioned by Cary Kaufman, it is very important to have the different specialties available, working in a multidisciplinary and integrated way
- A sufficient number of cases, to provide experience and continuing expertise
- Care by breast specialists in all of the required disciplines
- Provision of all the necessary services, from genetics to prevention to treatment of primary breast cancer and advanced breast cancer, and also links to palliative care
- Patient support
- Data collection and audit.
The latest update of the EUSOMA requirements for a specialist breast centre (EJC 2013, 49: 3579–87) still emphasises being an integrated breast centre or unit, with multidisciplinary and specialised care provided in an integrated way. In terms of numbers, the consensus is that a centre should see at least 150 newly diagnosed cases of primary breast cancer (all ages and stages) each year, covering a population of about 250,000 inhabitants. A breast surgeon must perform at least 50 breast surgeries, so a larger centre with more than three surgeons will need to see a higher volume than 150 newly diagnosed patients each year to provide each specialist with an adequate number. Centres must provide services throughout the patient pathway and also ensure data collection and audit.
There is growing discussion about providing continuity of care for patients with advanced or metastatic breast cancer, and also what competences are needed to provide a multidisciplinary approach for these patients. European accreditation systems are focused on primary breast cancer, but we also need to develop good quality indicators for advanced and metastatic breast cancer.
The services provided do not necessarily all have to be centralised in one breast centre. For example, if you have two breast centres in the same area, you might decide that you need only one radiation oncology department, and some centres may decide to outsource some other service(s). However, all decisions must be made by the multidisciplinary team of the centre where the patient is being treated.
What’s the definition of the multidisciplinary team? The new EUSOMA recommendations describe a ‘core team’ that includes a radiologist, radiographer, surgeon, reconstructive surgeon, pathologist, medical oncologist, radiation oncologist, breast care nurse and data manager, with specific requirements about the percentage of time each dedicates to breast care. The ‘non-core’ team are other specialists who are also important, but not necessarily part of the ‘core’ team, including: nuclear medicine specialists, gynaecologists, psycho-oncologists and clinical geneticists. In my breast unit, both the psycho-oncologist and nuclear medicine specialist are part of the ‘core team’, but this differs from centre to centre.
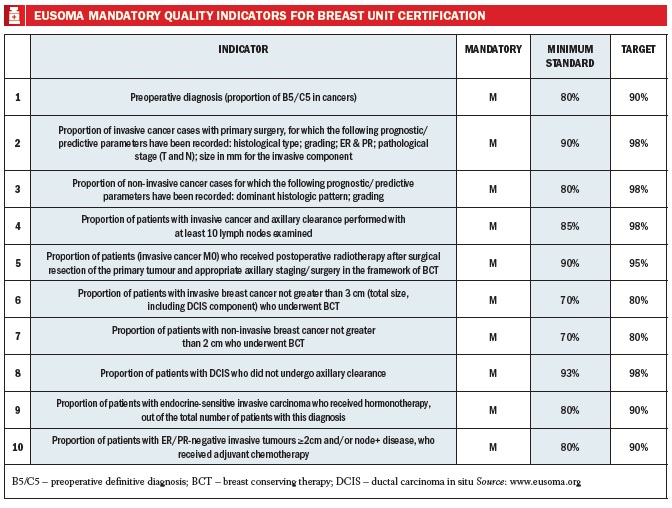
Quality indicators
There are 10 mandatory quality indicators for breast unit certification, each with a minimum standard and also an ideal standard (see table below). So taking as an example: ‘What is the optimal percentage of breast conserving therapy?’, EUSOMA recommends a minimum of 70%, although the target is 80%. Of course, this depends on the location, the country, and the availability of reconstructive surgery and radiation oncologists. But in breast centres that have all of these specialties, the target is 70–80% for breast conserving surgery.
Breast Centres Network
We go to all the effort of being accredited and ensuring quality care is established and appropriately implemented in centres, but how can we give this information to the public and the patients?
I was recently discussing this with European advocacy groups and they made the point that this information needs to reach people before they develop cancer, because when patients first receive a diagnosis they feel lost and it is not the best time to select a breast centre to go for treatment.
The European School of Oncology has developed the Breast Centres Network, which is the first international network of clinical centres for breast cancer. Every breast centre in Europe can enter their information in a standardised way, and indicate whether they are EUSOMA accredited or have other accreditation. The voluntary network website is user friendly and can be accessed by anyone, so a patient or member of the public can search for information on breast centres and their level of accreditation in their own country.
The European Commission and the European Parliament have also been working on this issue.
In 2003 the European Parliament noted that all breast cancer patients should be treated in a specialised breast unit, and recognised the need for a multidisciplinary approach. More recently, it has approved a resolution that by 2016 member states should have enshrined in law that all breast cancer patients are treated in a specialised breast centre or unit. Unfortunately, this is not yet in place in the majority of European countries, so this provision must be fought for at the level of individual countries.
Alongside this resolution, the European Commission has started a guidelines and accreditation project, aiming to incorporate the best breast cancer guidelines available in Europe, develop quality indicators and then establish an accreditation system that will be common to all European countries. This will still be a voluntary accreditation system, which has pros and cons, but it will cover all cancer services from prevention, screening and early detection to palliative care, so will be a very important effort. I hope that in two years’ time we will have another e-grand-round discussion about how the project has been implemented in all European countries.
The EU is establishing an accreditation system that will cover all cancer services, from prevention to palliative care
BOX 1- page 43 European School of Oncology e-grandround ESO presents fortnightly e-grandrounds which offer participants the chance to discuss a range of cutting-edge issues with leading European experts. One of these is selected for publication in each issue of Cancer World. In this issue, Cary Kaufman, chair of the US National Accreditation Program for Breast Centers, explains why an accreditation system for breast centers was introduced in the United States, and how it was done. Fatima Cardoso, EORTC secretary general and director of the breast unit at the Champalimaud Cancer Centre in Lisbon, Portugal, outlines the systems for breast centre accreditation in Europe and plans for the future. Edited by Susan Mayor. The recorded version of this and other e-grandrounds is available at www.e-eso.net





Leave a Reply